Abstract
In human cells, the ELL family of transcription factors includes at least three members, which are all capable of stimulating the overall rate of elongation by RNA polymerase II by suppressing transient pausing by the enzyme at many sites along DNA. In this report, we identify the ELL-associated factors (EAF)1 and EAF2 as strong positive regulators of ELL elongation activity. Our findings provide insights into the structure and function of ELL family transcription factors, and they bring to light direct roles for the EAF proteins in regulation of RNA polymerase II transcription.
Keywords: elongation, mRNA synthesis, transcription, transcription factor
The gene encoding the RNA polymerase II (pol II) elongation factor eleven-nineteen lysine-rich in leukemia (ELL) was first characterized as a fusion partner of the mixed lineage leukemia (MLL) gene in the (11, 19)(q23;p13.1) translocation in acute myeloid leukemia (1). The ELL protein has been shown to be capable of interacting with pol II and increasing its rate of transcript elongation in vitro by suppressing transient pausing by the enzyme (2, 3). Consistent with a role for ELL in controlling transcript elongation in vivo, Drosophila ELL colocalizes with pol II at transcriptionally active sites on polytene chromosomes, and evidence suggests that mutations in the gene encoding Drosophila ELL may preferentially affect synthesis of long transcripts (4).
In addition to ELL, human cells have two ELL paralogs, ELL2 and ELL3, which can also stimulate the rate of elongation by pol II in vitro (5, 6). ELL and ELL2 are expressed in a wide variety of tissues in humans. ELL3, on the other hand, appears to be expressed predominantly in the testis (5, 6).
A variety of ELL-interacting proteins have been identified through two-hybrid screens and biochemical purifications. Two of these proteins, EAF1 and EAF2 (7, 8), have been shown to colocalize with ELL in the nucleus in a stippled pattern that resembles that of Cajal bodies, structures that are enriched in factors involved in transcription and mRNA processing (9). Interestingly, Cajal bodies are disrupted in cells carrying the MLL-ELL translocation (10).
Suggesting that interactions between ELL and EAF1 may contribute to the leukemic phenotype of cells expressing the MLL-ELL chimera, the C-terminal domains of EAF1 and EAF2 share similarity with AF4 and ENL, other MLL translocation partners (7, 8). In addition, expression of artificial MLL-EAF1 and MLL-EAF2 chimeras can immortalize hematopoetic progenitor cells and induce the development of acute myeloid leukemia in mice (7, 8). Finally, and consistent with the possibility that EAF1 and EAF2 could play a role in transcriptional regulation, their C-terminal regions function as transcriptional activation domains when fused to the GAL4 DNA binding domain (7, 8).
In this article, we identify the EAF1 and EAF2 proteins as positive regulators of ELL elongation activity. Our findings provide insights into the mechanism of action of ELL family transcription factors, and they bring to light direct roles for the EAF proteins in controlling the overall rate of transcript elongation by pol II.
Materials and Methods
Materials. Unlabeled ultrapure ribonucleoside 5′-triphosphates and [α-32P]CTP (400 mCi/mmol; 1 Ci = 37 GBq) were purchased from Amersham Pharmacia Biosciences. Recombinant RNasin ribonuclease inhibitor was obtained from Promega. Anti-FLAG (M2) monoclonal antibodies, anti-Myc (C-3956) rabbit polyclonal antibodies, anti-FLAG (M2) agarose, and anti-FLAG peptide were from Sigma. Light chain-specific anti-mouse antibodies were purchased from Bethyl Laboratories and labeled with Alexa Fluor 680 (Molecular Probes) according to the manufacturer's instructions.
Expression of Recombinant Proteins in Insect Cells. Wild-type human ELL and ELL2, human ELL mutants, and human EAF1and EAF2 containing N-terminal FLAG or c-Myc epitope tags were subcloned into pBacPAK8. Recombinant baculoviruses were generated with the BacPAK expression system (Clontech). Sf21 cells were cultured at 27°C in Sf-900 II SFM (Invitrogen), supplemented with 10% FCS/100 units/ml penicillin/100 μg/ml streptomycin. Flasks containing 1 × 108 Sf21 cells were infected with the recombinant baculoviruses. Forty-eight hours after infection, cells were collected and lysed in 15 ml of ice-cold buffer containing 50 mM Hepes-NaOH (pH 7.9), 0.5 M NaCl, 5 mM MgCl2, 0.2% Triton X-100, 20% (vol/vol) glycerol, 0.28 μg/ml leupeptin, 1.4 μg/ml pepstatin A, 0.17 mg/ml PMSF, and 0.33 mg/ml benzamidine. Lysates were centrifuged 100,000 × g for 30 min at 4°C.
Purification of Recombinant Proteins. FLAG-tagged proteins were purified from Sf21 cell lysates by anti-FLAG agarose immunoaffinity chromatography. Lysates from 1 × 108 cells were incubated with 0.5 ml anti-FLAG (M2) agarose beads for at least 12 h at 4°C. The beads were washed three times with Tris-buffered saline (TBS), and bound proteins were eluted from the beads with TBS containing 10% (vol/vol) glycerol and 0.3 mg/ml FLAG peptide. Where indicated, anti-FLAG agarose eluates prepared from Sf21 cells expressing recombinant FLAG-EAF1 were further purified by anion exchange HPLC. Eluates were adjusted to a conductivity equivalent to that of 0.05 M NaCl and applied to a 0.6 ml TSK DEAE-NPR HPLC column (Tosoh-BioSep) preequilibrated in buffer A [40 mM Tris·HCl, pH 7.9/1 mM EDTA/1 mM DTT/10% (vol/vol) glycerol] containing 0.1 M NaCl. The column was eluted with a 6-ml linear gradient from 0.1 to 0.5 M NaCl in buffer A, and 0.2-ml fractions were collected. Concentrations of recombinant proteins were estimated by using scion image software (Scion, Frederick, MD) to compare the relative intensity of Coomassie blue stained bands corresponding to full-length proteins to the intensity of bands corresponding to BSA standards after SDS/PAGE.
Preparation of Pol II and General Transcription Factors. Pol II (11) and TFIIH (rat δ, TSK-SP fraction, ref. 12) were purified as described from rat liver nuclear extracts. Recombinant yeast TATA-box binding protein (13) and rat TFIIB (rat α, ref. 14) were expressed in E. coli and purified as described. Recombinant TFIIE was prepared as described in ref. 3. Recombinant TFIIF was expressed in Sf21 insect cells coinfected with baculoviruses encoding 6-histidine-tagged RAP74 and RAP30. Forty-eight hours after infection, cells were washed four times with PBS and lysed in 40 mM Hepes-NaOH, pH 7.9/0.5% (vol/vol) Nonidet P-40/10% (vol/vol) glycerol/0.35 M ammonium sulfate. Extracts were clarified by centrifugation at 50,000 × g for 15 min, and TFIIF was purified from the supernatant by affinity chromatography by using a 5-ml Hi-Trap chelating column (Amersham Pharmacia Biosciences) charged with NiSO4 and equilibrated with buffer I (40 mM Hepes-NaOH, pH 7.6/25 mM 2-mercaptoethanol/20% glycerol/0.1 M NaCl) with 10 mM imidazole (pH 7.9). The column was washed with buffer I containing 10 mM imidazole (pH 7.9) and eluted with buffer I supplemented with 500 mM imidazole (pH 7.9). Peak fractions were pooled, dialyzed against buffer D [40 mM Hepes-NaOH, pH 7.9/1 mM DTT/10% (vol/vol) glycerol], and TFIIF was further purified by chromatography on a 3-ml TSK SP-5PW HPLC column (Tosoh Bioscience) with a 30-ml linear gradient from 0.1 M to 1 M KCl buffer D.
DNA Templates for Transcription. pDN-AdML (11) and pAd-GR220 (15, 16) plasmid DNAs were isolated from Escherichia coli by using the alkaline lysis method (17) without RNase A. Plasmid DNA was banded twice in CsCl-ethidium bromide density gradients. Oligo(dC)-tailed pAd-GR220 was prepared as described in ref. 15. For preparation of the EcoRI to NdeI pDN-AdML fragment used in promoter-specific transcription assays, pDN-AdML was digested with EcoRI and NdeI, and the reaction mixture was adjusted to 0.6 M NaCl and subjected to chromatography on 1 ml HiTrap Q HP (Amersham Pharmacia Biosciences) anion exchange column as described by Westman et al. (18). Fractions containing the EcoRI to NdeI fragment of pDN-AdML were pooled, ethanol precipitated, and resuspended in 10 mM Tris·HCl/1 mM EDTA, pH 8.0.
Promoter-Specific Transcription Assays. All transcription reaction volumes were 60 μl, and all incubations were performed at 28°C. Preinitiation complexes were assembled by preincubation of ≈20 ng of the EcoIRI to NdeI fragment of pDN-AdML, ≈10 ng of recombinant TFIIB, ≈10 ng of recombinant TFIIF, ≈7 ng recombinant TFIIE, ≈40 ng TFIIH, ≈20 ng recombinant TFIIF, and ≈0.01 unit of pol II in 20 mM Hepes-NaOH, pH 7.9/20 mM Tris·HCl, pH 7.9/60 mM KCl/2 mM DTT/0.5 mg/ml BSA/3% (wt/vol) polyvinyl alcohol (average molecular weight 30,000-70,000 Da)/3% (vol/vol) glycerol/8 units of RNasin/8 mM MgCl2 for 30 min. Pulse-chase reactions were performed as described in the legend to Fig. 1 and stopped by the addition of 60 μl of Stop buffer (10 mM Tris·HCl, pH 7.2/0.5 mM EDTA/0.3 M NaCl/0.4% SDS) and 20 μg of Proteinase K. Reaction products were ethanol precipitated, resuspended in 9 M urea, and resolved on a sequencing gel containing 6% polyacrylamide, 7 M urea, and 45 mM Tris-borate/1 mM EDTA, pH 8.3 (17). Radioactive transcripts were detected with a Molecular Dynamics Typhoon PhosphoImager.
Fig. 1.
Transcript elongation activity of ELL is stimulated by EAF1. (A) Diagram of EcoRI to NdeI fragment of pDN-AdML, which contains AdML promoter sequences from -50 to +10 inserted into the polylinker of pUC18 and extends 254 nt downstream of the transcriptional start site (+1). The sequence of the nascent transcript from +1to +15 is shown. (B) Diagram of the reaction protocol used in the experiment of C. GTFs, general transcription factors TATA-box binding protein, TFIIB, TFIIE, TFIIF, and TFIIF; A, ATP; G, GTP; C, CTP; U, UTP; 32PC, [α-32P]CTP (C) ELL and EAF1, both with N-terminal FLAG tags, were expressed in Sf21 insect cells and purified by anti-FLAG affinity chromatography as described in Materials and Methods. Promoter-specific transcription reactions were performed as described in Materials and Methods. Transcription was initiated by the addition of 50 μM ATP, 50 μM UTP, and 10 μCi [α-32P]CTP. After 30 min (lane 1), reactions were chased with 100 μM CTP and 1 μM GTP in the presence or absence of 1 pmol ELL and 0.5 pmol EAF1 as indicated (lanes 2-9). A14 indicates the position of the nascent transcript that is synthesized in the presence of ATP, CTP, and UTP and stalled at the A residue at +14. The black arrowhead indicates the position of the full-length, 254-nt runoff transcript. Numbers on the left indicate the position of ΦX174 HaeIII restriction fragments used as size markers.
Oligo(dC)-Tailed Template Transcription Assays. Transcription reactions were carried out in the presence of 20 mM Hepes-NaOH, pH 7.9/20 mM Tris·HCl, pH 7.9/60 mM KCl/2 mM DTT/0.5 mg/ml BSA/3% (wt/vol) polyvinyl alcohol (average molecular weight 30,000-70,000 Da)/3% (wt/vol) glycerol/8 units of RNasin/8 mM MgCl2/≈100 ng oligo(dC)-tailed pAd-GR220/≈0.01 unit pol II,/ribonucleoside triphosphates and transcription factors indicated in the figures. In the experiments of Figs. 3, 4, 5, reactions were preincubated for 30 min at 28°C before the addition of ribonucleoside triphosphates. Reactions were stopped after incubation at 28°C for the times indicated in the figures, and products were resolved on 6% polyacrylamide gels containing 7 M urea and 45 mM Tris-borate/1 mM EDTA, pH 8.3 (17) and detected with a Molecular Dynamics Typhoon PhosphoImager.
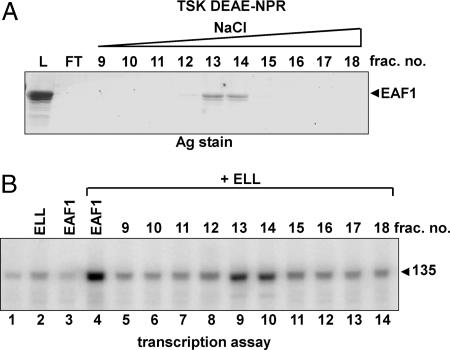
Fig. 3.
Cochromatography of ELL stimulatory activity with EAF1. (A) Approximately 15 μg of FLAG-EAF1, which had been purified by anti-FLAG agarose chromatography, was resolved on a 0.6 ml TSK DEAE-NPR HPLC column as described under Materials and Methods. The indicated fractions were resolved on a 12% SDS polyacrylamide gel and visualized by silver staining (Ag stain). (B) One-microliter aliquots of the indicated fractions were assayed for their ability to stimulate synthesis of 135-nt transcripts from the T-less cassette of oligo(dC)-tailed template pAd-GR220 in the presence of ELL (lanes 5 to 14). Controls (lanes 1 to 4) consist of reactions with pol II alone (lane 1) or supplemented with 1 pmol of the indicated proteins. Transcription was initiated by the addition of 50 μM ATP, 50 μM GTP, 2 μM CTP, and 10 μCi [α-32P]CTP (400 mCi/mmol) and stopped after a 5-min incubation.
Fig. 4.
EAF1-dependent stimulation of ELL elongation activity requires both the ELL N terminus and elongation activation domain. (A) Diagram of wild-type ELL and ELL mutants used in the experiments of B and C, showing the positions of the elongation activation (elongation), lysine-rich (K rich), and occludin-like domains. (B) Interaction of EAF1 and truncation mutant ELL proteins in Sf21 insect cells. Sf21 cells were infected with recombinant baculoviruses encoding the EAF1 protein with an N-terminal Myc tag, and the truncated ELL proteins were indicated with an N-terminal FLAG tag. Forty-eight hours after infection, cells were lysed as described in Materials and Methods and centrifuged at 20,000 × g for 30 min at 4°C. The resulting supernatants were subjected to immunoprecipitation with anti-FLAG agarose, and bound proteins were eluted from anti-FLAG beads with a 150 μg/ml FLAG peptide. Proteins present in anti-FLAG agarose eluates were fractionated by SDS/PAGE and visualized by Western blotting with anti-FLAG (red) or anti-myc (green) antibodies. (C) Two (lanes 2 and 3) or 8 pmol (lanes 5-11) of the indicated factors were assayed for their ability to stimulate synthesis of 135-nt transcripts from the T-less cassette of oligo(dC)-tailed template pAd-GR220. Transcription was initiated by the addition of 50 μM ATP, 50 μM GTP, and 10 μCi [α-32P]CTP (400 mCi/mmol), and reactions were incubated for 15 min.
Fig. 5.
Both EAF1 and EAF2 stimulate ELL elongation activity. Two picomoles of EAF1 or EAF2 was added to reactions with or without 1 pmol of either ELL or ELL2 as indicated. Transcription was initiated by addition of 50 μM ATP, 50 μM GTP, 2 μM CTP, and 10 μCi [α-32P]CTP and incubated for 5 min.
Results and Discussion
EAF1 Functions as a Positive Regulator of ELL Elongation Activity. To determine whether EAF1 affects the ability of ELL to stimulate elongation by pol II during promoter-specific transcription, we used a reconstituted transcription system composed of pol II and TFIIH purified from rat liver, and recombinant TATA-box binding protein, TFIIB, TFIIF, TFIIE, ELL, and EAF1. The DNA fragment used as a template for transcription includes nucleotides -50 to +10 from the Adenovirus 2 major late (AdML) promoter and extends 254 base pairs downstream from the transcriptional start site (Fig. 1 A). Transcription was initiated from the AdML promoter in the presence of pol II and the general transcription factors by addition of 50 μM ATP, 50 μM UTP, and 10 μCi [α-32P]CTP, which are sufficient for synthesis of 14-nt transcripts initiated at the AdML promoter (Fig. 1C, lane 1). Transcripts were then chased into longer products by the addition of 100 μM nonradioactive CTP and 1 μM GTP, in the presence or absence of ELL, EAF1, or both proteins. As expected, ELL alone was able to stimulate the rate of transcript elongation by pol II, as evidenced by an increase in the number of longer transcripts synthesized after both 5 and 10 min of chase (compare lanes 2 and 6 to lanes 4 and 8). EAF1 alone had no discernable effect on the rate of transcript elongation (lanes 3 and 7); however, EAF1 strongly stimulated the rate of transcript elongation when added to reactions that included ELL (lanes 5 and 9).
The experiment of Fig. 1 left open the possibility that functional interaction of EAF1 depends on the presence of one or more of the general transcription factors. To determine whether the ability of EAF1 to enhance ELL's elongation activity is independent of other transcription factors, we assayed transcript elongation on a linearized plasmid with a single-stranded 3′ oligo(dC)-tail on its template strand. Although purified pol II is unable to initiate from a specific location on double-stranded DNA without assistance from the general transcription factors, it binds the single-stranded oligo(dC)-tail and initiates transcription at the junction between the single- and double-stranded region of the template (19). On the template used in our experiments, the first nontemplate strand (dT) residue is 136 nt downstream of the oligo(dC)-tail, and the next run of (dT) residues is located from ≈240 to ≈250 residues from the oligo(dC)-tail. In the experiment of Fig. 2, transcription was initiated by the addition of purified pol II to reaction mixtures containing the oligo(dC)-tailed template, ATP, GTP, and [α-32P]CTP. After a 30-min incubation, labeled transcripts of ≈135 nt accumulated. These transcripts were then chased into longer products by the addition of a limiting concentration of UTP and an excess of nonradioactive CTP, in the presence and absence of ELL, EAF1, or both. Because the limiting concentration of UTP present during the chase lowers the rate of nucleotide addition through runs of (dT) residues, products between ≈240 and ≈250 nt in length accumulate and are eventually chased into longer products. Consistent with results of the promoter-specific assays, addition of EAF1 alone had no effect on the rate of transcript elongation. Addition of ELL alone led to a modest increase in the rate of transcript elongation, as detected by an increase in the rate at which the labeled 135-nt transcript was chased into products of ≈240-250 nt and longer, whereas addition of both EAF1 and ELL strongly stimulated the rate of transcript elongation by pol II.
Fig. 2.
EAF1 stimulates ELL elongation activity in the absence of additional transcription factors. Oligo(dC)-tailed template transcription reactions were initiated by the addition of 50 μM ATP, 50 μM GTP, 2 μM CTP, and 10 μCi [α-32P]CTP (400 mCi/mmol). After a 30-min incubation, 135-nt transcripts were chased into longer products in the presence of 2 μM UTP and 100 μM CTP, with or without 1 pmol ELL or 0.5 pmol EAF1 as indicated. Reaction products were analyzed as described for promoter-specific transcription assays. The lane marked M shows ΦX174 HaeIII restriction fragments used as size markers. To the right of the gel is diagrammed the oligo(dC)-tailed template used in these assays, showing the position of the oligo(dC)-tail (Cn)COH and the T rich stretches in the nascent transcript.
To determine whether EAF1, rather than some contaminant in our anti-FLAG agarose-purified FLAG-EAF1 preparation, was responsible for stimulating ELL elongation activity, we further fractionated anti-FLAG agarose-purified FLAG-EAF1 by TSK DEAE-NPR HPLC. FLAG-EAF1 bound the DEAE-NPR column at 0.1 M NaCl and eluted with ≈0.23 M NaCl, with the majority of the EAF1 protein detected by silver staining in fractions 13 and 14 (Fig. 3A). Fractions were then assayed for their ability to stimulate the rate of transcript elongation in the presence of a low concentration of ELL (Fig. 3B).
It is difficult to compare the relative activities of different enzyme fractions with the pulse-chase protocol used in Fig. 2, because the reaction gives rise to a collection of products with variable lengths rather than to a single, readily quantifiable species. Accordingly, in this and subsequent experiments, we have used a modified version of the tailed-template assay, in which we compare the rate of accumulation of the 135-nt, U-less transcript initiated from the oligo (dC)-tail on pAd-GR220 in the presence of only ATP, GTP, and a limiting concentration of CTP. During the short incubation time and limiting nucleotide concentrations used, only a fraction of pol II molecules are able to complete synthesis of the 135-nt transcript without assistance from elongation factors (2). As shown in the control experiments of Fig. 3B, lanes 1-4, ELL alone only very weakly stimulated accumulation of the 135-nt transcript, and FLAG immunopurified EAF1 alone had no effect; however, the mixture of ELL and FLAG-immunopurified EAF1 strongly stimulated accumulation of the 135-nt transcript. As shown in lanes 5-14, the activity that stimulated the rate of accumulation of 135-nt transcripts copurified precisely with EAF1 in fractions 13 and 14 from the DEAE-NPR HPLC column, arguing that EAF1, and not a contaminant, is responsible for enhancing ELL elongation activity. Taken together, the results presented thus far argue that the EAF1 protein is capable of stimulating ELL elongation activity directly, without the aid of additional transcription factors.
Positive Regulation of ELL Elongation Activity Depends on Stable Binding of EAF1 to the ELL N Terminus. Structure-function studies have shown that the region of ELL required for stimulation of elongation by pol II resides between ELL residues 60 and 373 (3), which are highly conserved between ELL and ELL2 (6). ELL and ELL2 also include a central lysine-rich region of unknown function, and a highly conserved occludin-like domain that had been shown to be important for the transforming activity of the MLL-ELL chimera (20, 21).
EAF1 has been shown to be able to interact physically with two distinct regions of ELL (7, 8). One of these regions falls within the ELL N terminus and includes residues 1-129, and the second falls within the ELL C terminus (7, 8) and includes the occludin-like domain. To determine which portions of ELL are needed for functional interaction of ELL with EAF1, we assessed the abilities of a series of ELL deletion mutants to interact with EAF1 in coimmunoprecipitation experiments. In these experiments, wild-type ELL or ELL deletion mutants containing N-terminal FLAG tags (Fig. 4A) were coexpressed in Sf21 insect cells with wild-type EAF1 containing an N-terminal c-Myc tag, and FLAG-ELL and FLAG-ELL mutants were purified by anti-FLAG agarose chromatography. Consistent with previous results (7, 8), EAF1 bound wild-type ELL or ELL mutants that include either the ELL N terminus or the ELL occludin-like domain; however, it did not bind ELL (45-373), which lacks both the N-terminal 44 amino acids and the occludin-like domain (Fig. 4B). Moreover, stimulation of ELL-dependent transcription by EAF1 strictly depends on the presence of the first 45 amino acids of ELL, even though this ELL region is not required for ELL's elongation activity in the absence of EAF1. As shown in oligo(dC)-tailed template assays of Fig. 4C, wild-type ELL, ELL (45-621), ELL (1-373), and ELL (45-373) increased accumulation of the 135-nt transcript above the low basal levels observed with pol II alone; however, a further increase in accumulation of the 135-nt transcript upon addition of EAF1 was observed only in reactions that contained wild-type ELL or ELL (1-373). As expected from previous results (3), the addition of EAF1 to transcription reactions containing ELL (500-621), which lacks the elongation activation domain, had no effect on accumulation of the 135-nt transcript (data not shown). Taken together, these results argue (i) that functional interaction between EAF1 and ELL requires not only the ELL elongation activation domain, but also the first 45 amino acids of ELL, and (ii) that ELL amino acids C-terminal to ELL's elongation activation domain are not needed for a transcriptional response to EAF1. They do not, however, exclude the possibility that the C-terminal end of ELL may have a transcriptional role in vivo.
The EAF1-Related EAF2 Protein Is also a Positive Regulator of ELL Elongation Activity. EAF2 is ≈60% identical and 75% similar in amino acid sequence to EAF1 (8). Furthermore, its interaction with ELL has been shown to depend on the first 45 amino acids of ELL (8), which we have shown are essential for functional interaction between EAF1 and ELL. We therefore asked whether EAF2 could also stimulate ELL elongation activity. As shown in Fig. 5, the addition of EAF1 or EAF2 to oligo(dC)-tailed template assays containing ELL or the closely related transcription elongation factor ELL2 markedly increased the accumulation of 135-nt transcripts. EAF2 had no effect on the accumulation of 135-nt transcripts when added to reactions lacking ELL or ELL2, arguing that, like EAF1, EAF2 functions as a positive regulator of ELL elongation activity.,
Summary and Perspective. In summary, in this report, we have identified the ELL-associated factors EAF1 and EAF2 as potent positive regulators of pol II elongation factors ELL and ELL2. Evidence from our mechanistic studies argues that the EAF proteins interact directly with ELL to form a stable complex that targets the pol II ternary elongation complex and strongly stimulates its overall rate of elongation. In addition, our experiments with the EAF1 protein argue that EAF1 stimulation of ELL elongation activity requires not only the ELL elongation activation domain (residues 45-373), but also the ELL N-terminal region (residues 1-45) that is lost in the oncogenic MLL-ELL fusion protein created by the (11, 19)(q23;p13.1) translocation in acute myeloid leukemia. Finally, the Xenopus laevis EAF2 protein has recently been shown to be required for Xenopus eye development. It functions in this process, at least in part, to activate transcription of the gene encoding the essential Rx homeodomain transcription factor (22). Our finding that the EAF proteins are capable of functioning together with the ELL family of transcription factors to expedite pol II transcription suggests that ELL contributes to the role of Xenopus EAF2 protein in eye development. Future studies exploiting the Xenopus Rx gene as a model EAF target gene should provide further insights into the mechanisms of action of the EAF and ELL proteins in regulation of pol II transcription.
Acknowledgments
This work was supported in part by National Institutes of Health Grant R37 GM41628 (to R.C.C.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AdML, Adenovirus 2 major late; EAF, ELL-associated factor; pol II, RNA polymerase II.
References
- 1.Thirman, M. J., Levitan, D. A., Kobayashi, H., Simon, M. C. & Rowley, J. D. (1994) Proc. Natl. Acad. Sci. USA 91, 12110-12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shilatifard, A., Lane, W. S., Jackson, K. W., Conaway, R. C. & Conaway, J. W. (1996) Science 271, 1873-1876. [DOI] [PubMed] [Google Scholar]
- 3.Shilatifard, A., Haque, D., Conaway, R. C. & Conaway, J. W. (1997) J. Biol. Chem. 272, 22355-22363. [DOI] [PubMed] [Google Scholar]
- 4.Gerber, M., Ma, J., Dean, K., Eissenberg, J. C. & Shilatifard, A. (2001) EMBO J. 20, 6104-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller, T., Williams, K., Johnstone, R. W. & Shilatifard, A. (2001) J. Biol. Chem. 275, 32052-32056. [DOI] [PubMed] [Google Scholar]
- 6.Shilatifard, A., Duan, D. R., Haque, D., Florence, C., Schubach, W. H., Conaway, J. W. & Conaway, R. C. (1997) Proc. Natl. Acad. Sci. USA 94, 3639-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simone, F., Polak, P. E., Kaberlein, J. J., Luo, R. T., Levitan, D. A. & Thirman, M. J. (2001) Blood 98, 201-209. [DOI] [PubMed] [Google Scholar]
- 8.Simone, F., Luo, R. T., Polak, P. E., Kaberlein, J. J. & Thirman, M. J. (2003) Blood 101, 2355-2362. [DOI] [PubMed] [Google Scholar]
- 9.Gall, J. G., Bellini, M., Wu, Z. & Murphy, C. (1999) Mol. Biol. Cell 10, 4385-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polak, P. E., Simone, F., Kaberlein, J. J., Luo, R. T. & Thirman, M. J. (2003) Mol. Biol. Cell 14, 1517-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conaway, J. W. & Conaway, R. C. (1990) Science 248, 1550-1553. [DOI] [PubMed] [Google Scholar]
- 12.Conaway, J. W., Bradsher, J. N. & Conaway, R. C. (1992) J. Biol. Chem. 267, 10142-10148. [PubMed] [Google Scholar]
- 13.Conaway, J. W., Hanley, J. P., Garrett, K. P. & Conaway, R. C. (1991) J. Biol. Chem. 266, 7804-7811. [PubMed] [Google Scholar]
- 14.Tsuboi, A., Conger, K., Garrett, K. P., Conaway, R. C., Conaway, J. W. & Arai, N. (1992) Nucleic Acids Res. 20, 3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice, G. A., Kane, C. M. & Chamberlin, M. J. (1991) Proc. Natl. Acad. Sci. USA 88, 4245-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan, S., Aso, T., Conaway, R. C. & Conaway, J. W. (1994) J. Biol. Chem. 269, 25684-25691. [PubMed] [Google Scholar]
- 17.Sambrook, J., Fritsch, E. F. & Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Woodbury, NY), 2nd Ed.
- 18.Westman, E., Eriksson, S., Laas, T., Pernemalm, P. A. & Skold, S. E. (1987) Anal. Biochem. 166, 158-171. [DOI] [PubMed] [Google Scholar]
- 19.Kadesch, T. R. & Chamberlin, M. J. (1982) J. Biol. Chem. 257, 5286-5295. [PubMed] [Google Scholar]
- 20.DiMartino, J. F., Miller, T., Ayton, P. M., Landewe, T., Hess, J. L., Cleary, M. L. & Shilatifard, A. (2000) Blood 96, 3887-3893. [PubMed] [Google Scholar]
- 21.Luo, R. T., Lavau, C., Du, C., Simone, F., Polak, P. E., Kawamata, S. & Thirman, M. J. (2001) Mol. Cell. Biol. 21, 5678-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurus, D., Héligon, C., Bürger-Schwärzler, A., Brändlli, A. W. & Kühl, M. (2005) EMBO J. 24, 1181-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]