Abstract
Leukemogenic viruses like human T-lymphotropic virus and bovine leukemia virus (BLV) presumably persist in the host partly by latent integration of the provirus in a fraction of infected cells, leading to accumulative increase in the outgrowth of transformed cells. Furthermore, viral infection also correlates with a blockade of the apoptotic mechanisms concomitant with an apparent latency of the host cell. Conceptually, induction of viral or cellular gene expression could thus also be used as a therapeutic strategy against retroviral-associated leukemia. Here, we provide evidence that valproate, an inhibitor of deacetylases, activates BLV gene expression in transient transfection experiments and in short-term cultures of primary B-lymphocytes. In vivo, valproate injection into newly BLV-inoculated sheep did not abrogate primary infection. However, valproate treatment, in the absence of any other cytotoxic drug, was efficient for leukemia/lymphoma therapy in the sheep model leading to decreased lymphocyte numbers (respectively from 25.6, 35.7, and 46.5 × 103 cells per mm3 to 1.0, 10.6, and 24.3 × 103 cells per mm3 in three leukemic sheep) and tumor regression (from >700 cm3 to undetectable). The concept of a therapy that targets the expression of viral and cellular genes might be a promising treatment of adult T cell leukemia or tropical spastic paraparesis/human T-lymphotropic virus-associated myelopathy, diseases for which no satisfactory treatment exists so far.
Keywords: gene activation therapy, histone deacetylase, immune response
The lymphoproliferative diseases caused by human T-lymphotropic virus (HTLV) and bovine leukemia virus (BLV) are characterized by an imbalance in the equilibrium between proliferation and death leading to the progressive accumulation of cells harboring an integrated provirus (1-3). Intriguingly, the great majority of the infected lymphocytes do apparently not express any viral protein in the peripheral blood (at least in the absence of culture) and rest in the G0/G1 phase of the cell cycle (3-5). These apparently quiescent cells may undergo spontaneous cell proliferation and express virions upon transient short-term culture. Viral encoded accessory proteins either activate (i.e., Tax, BLV G4) or repress (Rex, HTLV p30II) viral gene expression (3, 6). Whereas Tax activates expression at the level of transcription, Rex is required for the synthesis of the genomic and single-spliced envelope mRNAs. The HTLV-1-encoded p30II is a nuclear-resident protein that binds to, and retains in the nucleus, the doubly spliced mRNA encoding the Tax and Rex proteins (6). It is likely that an inhibitory mechanism hampers viral gene expression and provokes cell quiescence in vivo. Infected cell persistence would thus be permitted under the restrictive condition that the virus is not expressed (7). Evidence for a very strong immune response is supported by the presence of specific cytotoxic T cells and by high antiviral antibody titers. However, the lack of viral expression in a large proportion of infected cells hampers efficient clearance by the immune system. Concomitantly, virus infection also correlates with inhibition of the apoptotic processes, generating a reservoir of apparently latent cells (7-9). These quiescent lymphocytes either remain in the peripheral blood or transit through the lymphoid organs in the absence of any proliferation or protein expression.
In this context, we aimed at evaluating the therapeutic effectiveness of a strategy based on the induction of viral and cellular gene expression. Among a number of methodological approaches, modulation of chromatin condensation, which is an essential component of the gene expression pattern, can be achieved by interference with the level of histone acetylation (10-14). This process results from an intrinsic balance between the activity of two families of antagonistic enzymes, histone deacetylases (HDACs) and histone acetyl-transferases, respectively removing or incorporating acetyl groups into core histones. Acetyl removal by HDACs restores a positive charge to the lysine residues in the histone N-terminal tails and is thought to increase the affinity of histones for DNA, leading to transcriptional repression. Conversely, impairment of HDAC function with specific HDAC inhibitors (HDACi) activates both cellular and viral gene transcription (15, 16). In this context, BLV expression is increased by trichostatin A (TSA) in reporter-based assays as well as during short-term cultures of primary cells isolated from infected animals (17). The generalized use of TSA in vivo is however hampered by its potential toxicity, and, among a growing list of deacetylase inhibitors, valproate (the sodium salt of 2-propylpentanoic acid) offers a series of advantages (18-20). Known since several decades for the treatment of epilepsy, this short-chain fatty acid exhibits very low toxicity in adults and, with a half life of 16-17 h, has suitable pharmacokinetic properties in vivo (21, 22). Therefore, valproate provides a potential convenient tool to evaluate the effectiveness of a gene activation chemotherapy in a model of retroviral infection.
Materials and Methods
Luciferase-Based Reporter Assays. Human HeLa (epithelium-like cervix carcinoma) cells were cultured at a density of 2.5 × 105 per 10-cm2 well and transfected by using GeneJammer reagent (Stratagene) with 1 μg of pGL3-LTRWT reporter plasmid containing the BLV LTR promoter cloned upstream of the luciferase gene. To assess Tax-dependent transactivation, cells were also cotransfected either with 10 ng of pSGTax expressing the BLV Tax protein or with the pSG5 control expression vector. Jurkat T lymphocytes (4 × 106 cells resuspended in 5 ml of medium) were transfected with 2 μgof pGL3-LTRWT together with 10 ng of pSGTax or pSG5 by using Xtreme (Roche). Cells were then cultivated during 24 h in the presence of different concentrations of valproate (Sigma-Aldrich), washed with PBS, and analyzed for luciferase enzyme activity (17).
Ex Vivo Short-Term Cultures of Peripheral Blood Mononuclear Cells (PBMC). All BLV-infected (S1-S12) and control (C1-C5) were maintained under restricted conditions at the Veterinary and Agrochemical Research Center (Machelen, Belgium). The coordinates of the sheep are S1 (2158), S2 (2208), S3 (2213), S4 (2152), S5 (2153), S6 (2091), S7 (2675), S8 (4535), S9 (3002), S10 (2665), S11 (3003), C1 (4534), C2 (4533), C3 (2122), C4 (2127), and C5 (2147). At regular intervals of time, total leukocyte counts were determined by using a Coulter counter ZN, and the relative proportions of lymphocytes were estimated under the microscope after May-Grünwald Giemsa staining (Sigma-Aldrich). To determine the percentages of B lymphocytes, PBMC were labeled with anti-IgM monoclonal antibody (clones 1H4 or Pig45) in association with rat anti-mouse IgG1 phycoerythrin or goat anti-mouse IgG2b FITC conjugates (Caltag), respectively, and analyzed by flow cytometry (FACScan, Becton Dickinson). Ten thousand events were collected for each sample, and data were analyzed with the program cellquest (BD Immunocytometry Systems).
Venous blood was collected by jugular venipuncture and mixed with 0.3% wt/vol EDTA used as an anticoagulant. Then, PBMC were separated by Percoll density gradient centrifugation (Amersham Pharmacia Biosciences) and washed twice with PBS/0.075% EDTA and at least three times with PBS alone. After estimation of their viability by trypan blue dye exclusion, 2 × 106 cells were cultivated during 16 h at 37°C in complete RPMI medium 1640 (e.g., supplemented with 10% FCS/2 mM l-glutamine/100 units of penicillin/100 μg of streptomycin per ml; Invitrogen) in the presence or the absence of different concentrations of valproate (Sigma-Aldrich).
Analysis of ex Vivo Apoptosis. After 16 h of culture, the apoptotic rates in the PBMC were measured as described in ref. 17. Doublets were excluded from the analysis by using the (FL2a/FL2h/FL2w) gating method, and cells staining in sub-G1 were considered to be apoptotic.
Analysis of Viral Expression by Flow Cytometry and ELISA. To determine the number of cells expressing the p24 protein, sheep PBMC were cultivated during 16 h, fixed, and permeabilized by using DAKO IntraStain Reagent A and 1× Becton Dickinson Permeabilizing Solution 2. Intracellular detection of p24 was performed by sequential incubation with 4′G9 monoclonal antibody and a rat anti-mouse IgG1 phycoerythrin conjugate (Becton Dickinson) for 30 min at 4°C. Ten thousand events per sample were collected by flow cytometry and analyzed with cellquest.
To assess viral expression, PBMC and their corresponding supernatants were separated by centrifugation (10 min at 1.500 g) and analyzed for p24 protein synthesis by using an ELISA procedure (17). The optical densities were normalized to the levels obtained in the absence of valproate and corrected considering the proportion of nonapoptotic cells.
Experimental Design of Valproate Injection. Blood freshly collected from a BLV-infected sheep (S8) was injected both intravenously (5 ml) and s.c. (1 ml) into six sheep (S1-S6; total viral inoculum estimated at 16 × 106 infected cells). Ten grams of valproate (in 30 ml of sterile NaCl 0.9%) were injected intramuscularly in three BLV-infected sheep (S4, S5, and S6) as well as in age-matched control animals (C3, C4, and C5). Valproate injections were initiated the day before viral inoculation and continued thrice weekly over a 1-month period. This amount of valproate thus corresponds to a daily dose of ≈80 mg/kg per day, up to 250 mg/kg having been tested in sheep (23).
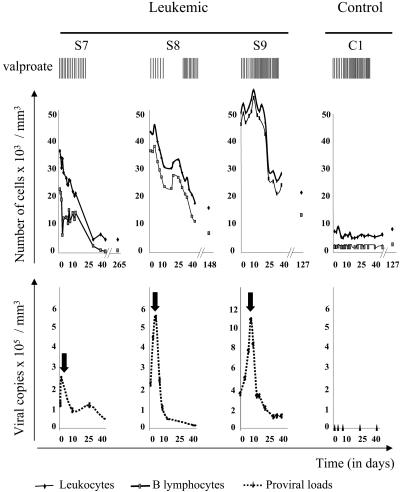
The leukemic sheep (S7, S8, and S9) and the control (C1) received i.v. injections of valproate (16, 18, 30, and 30 doses, respectively, of 10 g each) spread over 40 days, as indicated in the legend of Fig. 4 (vertical bars). Of note, sheep S8, S9, and C1 were splenectomized. For the treatment of animal S10, valproate was first infused directly into the prescapular tumor (two times, 10 g) and then intramuscularly (20 injections over 40 days). The volume of the tumor (in cm3) was regularly calculated by using the equation V = π·h·(3r2 + h2)/6, where r is the radius and h is the height.
Fig. 4.
Three leukemic sheep (S7, S8, and S9) and one control sheep (C1) received i.v. injections of valproate (10 g each) as indicated by the vertical bars (valproate). The absolute numbers of leukocytes (× 103 per mm3; black lines in Upper) were determined with a hematological counter, and the relative proportions of lymphocytes were estimated under the microscope after May-Grünwald Giemsa staining. The numbers of B cells per mm3 (gray lines in Upper) were determined by flow cytometry with anti-IgM antibody and normalized to the absolute lymphocyte counts. The proviral loads (represented as numbers of copies per mm3 of blood in Lower) were measured by real-time PCR with genomic DNA and were normalized with an 18S ribosomal amplification curve. The arrows in Lower indicate the peak in the proviral loads.
Determination of the Proviral Loads. Genomic DNA was extracted from an aliquot of blood (300 μl) containing 0.3% of EDTA as an anticoagulant. After disruption of the erythrocytes with a 3-fold excess of cell lysis buffer (Promega), the samples were digested overnight with RNase A (0.1 mg/ml) and proteinase K (0.2 mg/ml) in a buffer containing 100 mM Tris·HCl (pH 8), 150 mM NaCl, 10 mM EDTA, and 0.5% SDS. The DNA was next purified by a phenol-chloroform extraction and by ethanol precipitation. One hundred nanograms of genomic DNA were used for real-time PCR amplification of BLV proviral sequences essentially as described in ref. 24. A standard curve was generated after amplification of defined proviral copy numbers (from 1 to 107 of plasmid pBLV344) diluted in 100 ng of control genomic DNA. To correct for differences in DNA concentrations and amplification efficiencies between samples, the 18S ribosomal DNA was quantified in parallel as described in ref. 24. Under these conditions, the sensitivity of the technique was below one viral copy detected in 100 ng of DNA. The numbers of copies were finally normalized to the corresponding blood volume (in mm3).
Results
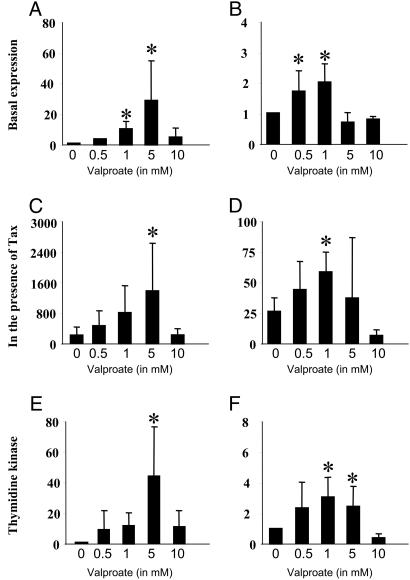
Valproate Enhances Viral-Promoter-Driven Transcription. We previously demonstrated that two highly specific deacetylase inhibitors, i.e., trichostatin A and trapoxin, activate transcription directed by the BLV promoter (17). Because these chemicals never progressed to the clinical stage, other deacetylase inhibitors were tested for their ability to induce LTR-driven expression in vitro. Among these, valproate, which combines deacetylase inhibitory properties with lack of toxicity in vivo, efficiently increased luciferase activity under the control of the LTR promoter (plasmid pGL3-LTRWT) in HeLa cells (Fig. 1A) as well as in Jurkat T lymphocytes (Fig. 1B). Furthermore, at pharmacologically relevant concentrations (0.5-5 mM), valproate also stimulated Tax-directed transactivation of the LTR (Fig. 1 C and D). Under identical experimental conditions, the thymidine kinase promoter known to be regulated by acetylation (11, 25) was also responsive to valproate in both cell lines, as expected (Fig. 1 E and F). As control, luciferase activities remained at background levels in cells transfected by an empty pGL3-basic reporter (data not shown).
Fig. 1.
Effect of valproate on BLV LTR and thymidine kinase promoter activity. HeLa cells (A and C) and Jurkat T lymphocytes (B and D) were transfected with 2 μg of the pGL3-LTRWT reporter, which contains the BLV LTR cloned upstream of the luciferase gene, in the absence (basal expression: A and B) or in the presence of 10 ng of plasmid pSGTax (in the presence of Tax: C and D). (E and F) HeLa and Jurkat cells were transfected with two micrograms of pGL2-TK in which the thymidine kinase promoter is inserted into the pGL2 reporter. Transfected cells were cultivated for 18 h without (0) or with valproate (0.5, 1, 5, and 10 mM) and luciferase activity was determined. The data (in arbitrary units ± standard deviation) result from three independent experiments. *, statistically significant P < 0.05 (Student's t test).
It thus appears that valproate efficiently activates transcription driven by the viral (e.g., BLV) and a cellular (thymidine kinase) promoter in vitro.
Valproate Is Proapoptotic and Increases the Level of BLV Expression ex Vivo. Despite an apparent lack of BLV protein expression in most cells containing an integrated provirus in vivo, some of them spontaneously recover their ability to produce viral particles when cultured ex vivo (26, 27). A generally accepted model postulates that this phenomenon occurs persistently in vivo but that the virus-positive cells are quickly cleared by the immune system. Furthermore, the B lymphocytes that express viral proteins do not undergo programmed cell death during ex vivo short-term cultures, suggesting an inhibition process of apoptotic death (28).
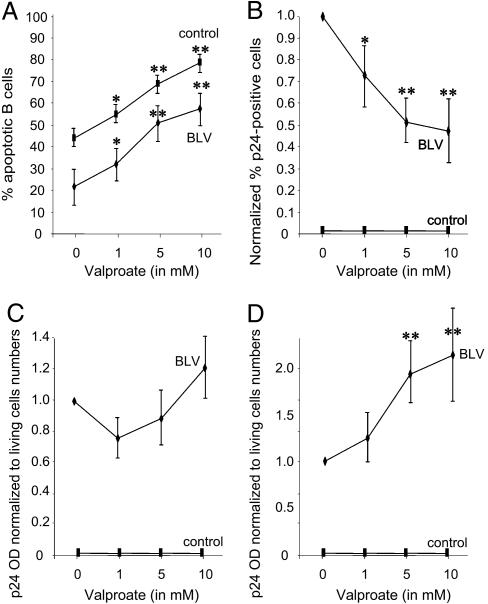
To assess the role of valproate in cell apoptosis and viral expression, PBMC were isolated from BLV-infected sheep (asymptomatic S1, S2, and S3, and lymphocytic/leukemic S8, S9, and S11) and control animals (C2, C3, C4, and C5). In the absence of valproate, the percentages of apoptotic B lymphocytes determined by a DNA fragmentation assay were lower in infected cell cultures compared with the controls (Fig. 2A, 0 mM valproate), illustrating an inhibition of cell death. Increasing concentrations of valproate triggered apoptosis in a dose-dependent manner both in infected and control B lymphocytes (1, 5, and 10 mM; Fig. 2A). Concomitantly, the proportions of cells expressing the major p24 viral capsid antigen, as measured by flow cytometry, gradually decreased in the presence of valproate (Fig. 2B). Under the same experimental conditions, intracellular p24 protein synthesis estimated by ELISA and normalized to the proportions of living cells was only marginally affected (Fig. 2C). In contrast, p24 expression significantly augmented in the culture medium, indicating enhanced virion production in the presence of valproate (Fig. 2D). Finally, Western blot analysis of the cultured cells confirmed the hyperacetylation of histone H3 upon valproate treatment (data not shown).
Fig. 2.
PBMC were isolated from six BLV-infected sheep (S1, S2, S3, S8, S9, and S11) and four control animals (C2, C3, C4, and C5) and cultivated during 16 h in the absence or the presence of different concentrations of valproate (from 0, 1, 5, and 10 mM). The percentages of cells undergoing apoptosis (A) or expressing p24 (B) were determined by flow cytometry. The amount of p24 major capsid antigen inside the cells (C) and in the supernatant (D) was measured by ELISA. In B, the data were normalized to the levels measured in the absence of valproate arbitrarily set to 1. In C and D, the net optical densities as determined by ELISA were normalized to the proportions of living cells as well as to the values obtained in the absence of valproate. The results are the means of five independent experiments. *, P < 0.05; **, P < 0.01 (statistically significant and highly statistically significant as determined by Student's t test). For additional controls, see Fig. 8, which is published as supporting information on the PNAS web site.
Collectively, the data show that valproate triggers viral expression and induces apoptosis of primary infected lymphocytes.
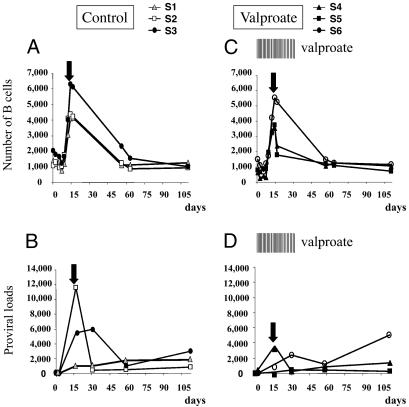
Valproate Treatment Does Not Prevent Primary Infection. As an activator of viral expression in vitro and ex vivo, valproate could thus provide a means for evaluating the effectiveness of a gene activation chemotherapy in an animal model. For this purpose, a first experiment was designed to test the effect of a valproate treatment upon experimental infection of sheep. Although valproate has low toxicity in human (at least in adults at 20 mg/kg per day, the maximal advised amount being 60 mg/kg per day) (29, 30), a preliminary experiment first verified the innocuousness of an 80 mg/kg per day dose in three control sheep during 1 month (i.e., 10 g of valproate every 2 days in animals C3, C4, and C5 of ≈60-65 kg weight). Besides an increase in the number of neutrophils (typically up to 4-fold at day 2), no major toxicity or side effects were observed (data not shown). Next, with the aim of impeding viral transmission, the same protocol was used for treatment of sheep inoculated with BLV-infected blood. Primary infection of sheep S1, S2, and S3 correlated with a transient accumulation of B lymphocytes peaking at the seroconversion period, i.e., the time at which BLV-specific neutralizing antibodies appear in the serum (arrow in Fig. 3A) (31). Active viral replication also occurs during this period, as revealed by an increase in the proviral loads (Fig. 3B). Upon treatment with valproate, the extent of B cell lymphocytosis as well as the onset of seroconversion were similar in the three BLV-inoculated sheep, indicating that infection did occur in these animals (S4, S5, and S6; Fig. 3C). Serial valproate injections reduced the proviral loads, but the difference with the untreated controls was not significant (Fig. 3 B and D).
Fig. 3.
A dose equivalent to 16 × 106 p24-positive cells from a BLV-infected sheep (S8) was injected in a series of six sheep (S1, S2, and S3 in A and B, and S4, S5, and S6 in C and D). In the three latter, valproate (10 g in NaCl 0.9%) injections were initiated 1 day before virus inoculation and continued thrice weekly over a 1-month period (vertical bars, valproate). The numbers of B cells per mm3 (A and C) were determined by flow cytometry with anti-IgM antibody and normalized to the absolute lymphocyte counts. The day of seroconversion (black arrows) corresponds to the onset of a BLV-specific humoral response. The proviral loads (represented as numbers of copies in 100 ng of DNA; B and D) were measured by real-time PCR with ovine genomic DNA normalized with an 18S ribosomal amplification curve.
It thus appears that, under the experimental conditions used, valproate therapy correlates with a slight reduction of the average viral loads but does not prevent primary infection of sheep.
Leukemia Therapy with Valproate. In the absence of satisfactory treatment for HTLV-induced leukemia, valproate offers the opportunity to evaluate the efficiency of a novel therapy in an animal model. With this aim, three BLV-infected sheep (S7, S8, and S9) at the leukemic phase of the disease (25.6, 35.7, and 46.5 × 103 B lymphocytes, respectively, per mm3) received serial i.v. injections of valproate (10 g each; see vertical bars in Fig. 4). The leukocyte numbers as well as the B lymphocyte counts gradually decreased over a 40-day time period (Fig. 4 Upper). Unexpectedly, the cell numbers lessened even when the treatment was interrupted and the animals did not relapse (at days 265, 148, and 127 for sheep S7, S8, and S9, respectively; Fig. 4). Interestingly, the proviral loads, as expressed in number of copies per mm3 of blood, peaked soon after initiation of the treatment (see arrows indicating the peak in the proviral loads in Fig. 4 Lower) and regularly decreased thereafter. In contrast, in the noninfected control sheep (C1), the cell counts remained remarkably constant during the valproate treatment, indicating an apparent innocuousness of the therapy.
Collectively, these data illustrate an example of an efficient valproate-based therapy of a viral-induced leukemia in the sheep model.
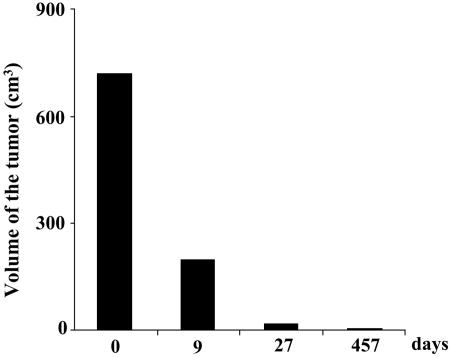
Valproate Is also Efficient for Lymphoma Therapy. Another clinical manifestation of BLV infection in sheep is lymphoma, a final and quickly lethal phase of the disease that might occur independently of leukemia. A typical example of this kind of tumor was provided by sheep S10 harboring a huge lymphoma (>700 cm3) in its prescapular lymph node. Valproate was first infused directly into the tumor (twice, 10 g) and then intramuscularly (20 injections over 40 days), the reason being that a massive inflammation within the lymphoma hampered subsequent injections. Interestingly, low but significant levels of viral p24 antigen (3.7 ng/liter) could be detected in the tumor exudation collected by needle biopsy, indicating that viral expression was induced in vivo. The tumor steadily regressed and did not reappear over a 15-month period in the absence of any further treatment (Fig. 5). At that time, a PCR amplification performed on a biopsy of the prescapular lymph node was negative for viral sequences (data not shown).
Fig. 5.
Valproate (10 g at days 1 and 2) was first infused directly into the prescapular tumor of sheep S10 and then intramuscularly (20 injections over 40 days). The tumor volume in cm3 was calculated at days 0, 9, 27, and 457 by using the equation V = π.h.(3r2 + h2)/6, where r is the radius and h is the height.
Discussion
Based on antitumor potential in cell lines and in xenografts, gene activation therapy with HDACi is a promising strategy against leukemia (12, 13, 32, 33). However, clinical development of these HDACi faces problems of ineffectiveness or major toxicity in vivo. Here, we provide evidence that the well known antiepileptic drug valproate is efficient in the absence of any other chemotherapy for treatment of leukemia and lymphoma induced by BLV in sheep. At the initiation of the therapy, all four animals were in the acute and progressive phase of the disease; two of them (S7 and S10) died from unrelated causes at 9 and 15 months posttreatment, another (S8) survived during 207 days, and the last one (S9) is still alive (see Fig. 6, which is published as supporting information on the PNAS web site).
The anticancer effect correlates with activation of viral expression ex vivo, suggesting the involvement of a clearance process operated by the immune system. This type of immune response could also be directed toward tumor antigens expressed by the host cell. Because valproate treatment is also effective against chronic lymphocytic leukemia cells not known to be virus-associated (unpublished data), other mechanisms might be implicated among which are modulation of surface molecules important for immune response (e.g., increased expression of MHC-I or IgG) or cellular matrix interactions (34, 35), increased sensitivity to the FasL/Fas signaling cascade (36) associated with NK activity (37), reversion of DNA methylation patterns (38) and proteosomal degradation of HDAC2 (19), targeting of the Wnt and ERK signaling pathways (39, 40), cell-cycle G1 arrest via reduction of cyclin D1 and D3 levels (41, 42), onset of apoptosis via caspases 8 and 9 (22) possibly by regulating the mitotic spindle checkpoint (43), and induction of differentiation (18) or inhibition of angiogenesis (44) (via vascular endothelial growth factor). Although the metabolic pathways incriminated in tumor-cell clearance by valproate in BLV-infected sheep remain to be characterized, our present data support a model based on the activation of viral expression possibly associated with replication and subsequent destruction by the immune system. Indeed, besides transcriptional activation of the LTR (Fig. 1) and induction of p24 synthesis in primary lymphocytes (Fig. 2), valproate administration in vivo transiently increases the proviral loads (Fig. 4). Our present working hypothesis postulates that these cells are very quickly destroyed via cytotoxic and humoral responses and cleared from the peripheral blood possibly during their transit through lymphoid organs such as the spleen or the lymph nodes. The requirement of a specific immune response (e.g., presence of high antibody titers as well as a CTL response) is further supported by the inability of valproate to abolish primary infection of sheep (Fig. 3). In this context, the innate immunity and maybe a proapoptotic activity associated with valproate might, however, be sufficient to partly reduce the proviral loads. Importantly, valproate treatment did not increase BLV replication during early infection, consistent with the idea that BLV replication is not limited by latency or by the host immune response. In contrast, transient viral replication apparently occurs upon injection of valproate in leukemic sheep (see proviral loads in Fig. 4). Indeed, normalization of the proviral loads to the absolute numbers of cells reveals an excess of viral copies (i.e., more than one copy per cell) that might result from multiple infections of the same cell or from amplification of unintegrated viral intermediates, indicating that BLV replication is initiated by the valproate treatment. An alternative but less likely model postulates a massive mobilization of infected lymphocytes from the lymphatic system into the periphery with concomitant exit of normal cells.
Although valproate was efficient for leukemia and lymphoma therapy, all sheep remained persistently infected; therefore, continuous or multiple valproate injections might be required to eradicate all infected cells (i.e., those susceptible to produce the virus). For example, it can be hypothesized that methylated provirus will remain silent after valproate treatment. However, in treated sheep, the number of leukemic cells continuously decreased even after interruption of the valproate perfusions [at week 3 for animal S7 (see Fig. 4) and at days 71 and 60 for sheep S8 and S9, respectively]. This quite unexpected result significantly differs from classical chemotherapy, which is directly cytotoxic for the leukemic cells and thus only during the treatment. Because the half-life of valproate in the plasma is ≈16-17 h, it thus appears that cells may die long after having been in contact with the HDACi (see Tables 1 and 2, which are published as supporting information on the PNAS web site). We speculate that destruction of these late-dying cells requires a second event, e.g., an immune stimulation in secondary lymphoid organs triggering cell proliferation. In terms of cell dynamics, the elimination of rarely proliferating cells would thus also require extended periods of time.
Although the strategy proposed in this report is promising, its potential application to human HTLV-1-induced leukemia (adult T cell leukemia) might encounter several restrictions, such as deletion of proviral sequences preventing viral gene expression, transient but excessive accumulation of provirus-positive lymphocytes and neutrophils, or interference with other immunosuppressive pathogens (HIV). Furthermore, the well characterized and rather safe pharmacokinetics determined for epilepsy might need to be adapted for leukemia therapy [daily doses of 25-60 mg/kg per day may be administered to patients (29, 30)]. Although lower amounts might be sufficient, a daily dose of 5 g (10 g every other day equivalent to 80 mg/kg per day) was effective in leukemic sheep. Although these parameters and limitations should thus be evaluated directly in leukemic patients, we would however propose a strategy based on a valproate-dependent gene activation process, possibly in combination with classical cytotoxic chemotherapy. Importantly, in malignant cell cultures, optimal cell killing is obtained when HDACi treatment precedes chemo- or radiotherapy. For example, treatment with deacetylase inhibitors up-regulates the hormone receptor on breast cancer cells and restores their susceptibility to tamoxifen therapy (45).
In regard with its pleiotropic effects, a final point to be discussed concerns the surprising innocuousness of the valproate HDACi toward normal cells in vivo. Indeed, serial valproate injections were toxic neither to B lymphocytes of control sheep (Fig. 4) nor to other cell types (e.g., CD4, CD8, and γδ) in the leukemic animals (see Fig. 7, which is published as supporting information on the PNAS web site). In the absence of apparent specificity, the selectivity of the antitumoral effect of valproate might be conferred by defined defects in the mitotic checkpoints of the transformed cells (43). Alternatively, the lack of general toxicity would rely on the capacity of normal lymphocytes to recover a quiescent stage, whereas in leukemic cells, the release of the apoptotic block would restore their dying ability. This mechanism would also explain why valproate is only partly active against bovine persistent lymphocytosis, a benign stage of the pathogenesis characterized by an accumulation of nontransformed cells (data not shown).
In conclusion, we have demonstrated here the feasibility of a gene-activation therapy in the context of a leukemia induced by BLV and broadened the scope of observations recently obtained in mice (46, 47). This strategy is also promising against other tumors like canine basal cell carcinoma, which responds at least partially to valproate treatment (A.J. and M.R., unpublished work). More importantly, short-term cultures of primary cells also support the possibility to treat chronic lymphocytic leukemia, one of the most prominent forms of human leukemia (L.L., unpublished results). And finally, valproate, perhaps in combination with other chemotoxic drugs promoting apoptosis of preactivated cells, might be instrumental for HTLV-induced adult T cell leukemia or perhaps tropical spastic paraparesis for which no satisfactory treatment exists so far.
Supplementary Material
Acknowledgments
We thank Charles Bangham (Imperial College London) for very critical reading of the manuscript and for suggestions. The valproate plasmatic levels were performed with the help of D. Gnat, F. Vertongen, and C. Mascaux (Jules Bordet Institute). We thank A. De Wilde, A. Drapier, J. M. Londes, C. Parent, A. Pary, V. Suin, G. Vandendaele, and M. Zaborna for experimental assistance and J. J. Letesson (Facultés Universitaires Notre Dame de la Paix, Namur, Belgium), D. Portetelle (Gembloux University Faculty of Agronomic Sciences), and K. Walravens (Veterinary and Agrochemical Research Center) for providing reagents. A.A., N.G., and P.U. (Télévie Fellows), A.F. (Research Fellow), C.D. (Postdoctoral Researcher), L.L. (Research Associate), and R.K. and L.W. (Research Directors) are members of the Fonds National de la Recherche Scientifique (FNRS). This work was supported by the Fondation contre le Cancer, the Fonds National de la Recherche Scientifique, the Fortis Bank Assurance, the Interuniversity Attraction Poles Program-Belgian Science Policy P4/30, and the Commissariat Général aux Relations Internationales, Direction Générale des Relations Extérieures (Région Wallonne).
Author contributions: L.W. designed research; A.A., A.F., N.G., C.D., P.U., F.V., A.J., M.R., P.K., and L.L. performed research; A.F., N.G., and G.M.F. contributed new reagents/analytic tools; A.B. and L.W. analyzed data; and L.W. wrote the paper.
Abbreviations: BLV, bovine leukemia virus; HDAC, histone deacetylase; HDACi, HDAC inhibitor(s); HTLV, human T-lymphotropic virus; PBMC, peripheral blood mononuclear cells.
References
- 1.Gallo, R. C. (2002) Immunol. Rev. 185, 236-265. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida, M. (2001) Annu. Rev. Immunol. 19, 475-496. [DOI] [PubMed] [Google Scholar]
- 3.Willems, L., Burny, A., Collete, D., Dangoisse, O., Dequiedt, F., Gatot, J. S., Kerkhofs, P., Lefebvre, L., Merezak, C., Peremans, T., et al. (2000) AIDS Res. Hum. Retroviruses 16, 1787-1795. [DOI] [PubMed] [Google Scholar]
- 4.Hanon, E., Asquith, R. E., Taylor, G. P., Tanaka, Y., Weber, J. N. & Bangham, C. R. M. (2000) AIDS Res. Hum. Retroviruses 16, 1711-1715. [DOI] [PubMed] [Google Scholar]
- 5.Moritoyo, T., Izumo, S., Moritoyo, H., Tanaka, Y., Kiyomatsu, Y., Nagai, M., Usuku, K., Sorimachi, M. & Osame, M. (1999) J. Neurovirol. 5, 241-248. [DOI] [PubMed] [Google Scholar]
- 6.Nicot, C., Dundr, M., Johnson, J. M., Fullen, J. R., Alonzo, N., Fukumoto, R., Princler, G. L., Derse, D., Misteli, T. & Franchini, G. (2004) Nat. Med. 10, 197-201. [DOI] [PubMed] [Google Scholar]
- 7.Bangham, C. R. (2003) J. Gen. Virol. 84, 3177-3189. [DOI] [PubMed] [Google Scholar]
- 8.Jeang, K. T., Giam, C. Z., Majone, F. & Aboud, M. (2004) J. Biol. Chem. 279, 31991-31994. [DOI] [PubMed] [Google Scholar]
- 9.Lum, J. J. & Badley, A. D. (2003) Curr. HIV Res. 1, 261-274. [DOI] [PubMed] [Google Scholar]
- 10.Curtin, M. & Glaser, K. (2003) Curr. Med. Chem. 10, 2373-2392. [DOI] [PubMed] [Google Scholar]
- 11.Dressel, U., Renkawitz, R. & Baniahmad, A. (2000) Anticancer Res. 20, 1017-1022. [PubMed] [Google Scholar]
- 12.McLaughlin, F., Finn, P. & La Thangue, N. B. (2003) Drug Discovery Today 8, 793-802. [DOI] [PubMed] [Google Scholar]
- 13.Momparler, R. L. (2003) Oncogene 22, 6479-6483. [DOI] [PubMed] [Google Scholar]
- 14.Secrist, J. P., Zhou, X. & Richon, V. M. (2003) Curr. Opin. Investig. Drugs 4, 1422-1427. [PubMed] [Google Scholar]
- 15.Thiagalingam, S., Cheng, K. H., Lee, H. J., Mineva, N., Thiagalingam, A. & Ponte, J. F. (2003) Ann. N.Y. Acad. Sci. 983, 84-100. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida, M., Shimazu, T. & Matsuyama, A. (2003) Prog. Cell Cycle Res. 5, 269-278. [PubMed] [Google Scholar]
- 17.Merezak, C., Reichert, M., van Lint, C., Kerkhofs, P., Portetelle, D., Willems, L. & Kettmann, R. (2002) J. Virol. 76, 5034-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottlicher, M., Minucci, S., Zhu, P., Kramer, O. H., Schimpf, A., Giavara, S., Sleeman, J. P., Lo Coco, F., Nervi, C., Pelicci, P. G., et al. (2001) EMBO J. 20, 6969-6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer, O. H., Zhu, P., Ostendorff, H. P., Golebiewski, M., Tiefenbach, J., Peters, M. A., Brill, B., Groner, B., Bach, I., Heinzel, T., et al. (2003) EMBO J. 22, 3411-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phiel, C. J., Zhang, F., Huang, E. Y., Guenther, M. G., Lazar, M. A. & Klein, P. S. (2001) J. Biol. Chem. 276, 36734-36741. [DOI] [PubMed] [Google Scholar]
- 21.Blaheta, R. A., Nau, H., Michaelis, M. & Cinatl, J. (2002) Curr. Med. Chem. 9, 1417-1433. [DOI] [PubMed] [Google Scholar]
- 22.Kawagoe, R., Kawagoe, H. & Sano, K. (2002) Leuk. Res. 26, 495-502. [DOI] [PubMed] [Google Scholar]
- 23.Wong, H., Rurak, D. W., Kumar, S., Kwan, E., Abbott, F. S. & Riggs, K. W. (2001) Drug Metab. Dispos. 29, 664-675. [PubMed] [Google Scholar]
- 24.Lew, A. E., Bock, R. E., Molloy, J. B., Minchin, C. M., Robinson, S. J. & Steer, P. (2004) J. Virol. Methods 115, 167-175. [DOI] [PubMed] [Google Scholar]
- 25.Schuettengruber, B., Doetzlhofer, A., Kroboth, K., Wintersberger, E. & Seiser, C. (2003) J. Biol. Chem. 278, 1784-1793. [DOI] [PubMed] [Google Scholar]
- 26.Kettmann, R., Marbaix, G., Cleuter, Y., Portetelle, D., Mammerickx, M. & Burny, A. (1980) Leuk. Res. 4, 509-519. [DOI] [PubMed] [Google Scholar]
- 27.Lagarias, D. M. & Radke, K. (1989) J. Virol. 63, 2099-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dequiedt, F., Hanon, E., Kerkhofs, P., Pastoret, P. P., Portetelle, D., Burny, A., Kettmann, R. & Willems, L. (1997) J. Virol. 71, 630-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephen, L. J. (2003) Drugs Aging 20, 141-152. [DOI] [PubMed] [Google Scholar]
- 30.Yu, K. T., Mills, S., Thompson, N. & Cunanan, C. (2003) Epilepsia 44, 724-726. [DOI] [PubMed] [Google Scholar]
- 31.Debacq, C., Sanchez Alcaraz, M. T., Mortreux, F., Kerkhofs, P., Kettmann, R. & Willems, L. (2004) Retrovirology 1, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marks, P. A., Rifkind, R. A., Richon, V. M. & Breslow, R. (2001) Clin. Cancer Res. 7, 759-760. [PubMed] [Google Scholar]
- 33.Blaheta, R. A. & Cinatl, J. (2002) Med. Res. Rev. 22, 492-511. [DOI] [PubMed] [Google Scholar]
- 34.Maeda, T., Towatari, M., Kosugi, H. & Saito, H. (2000) Blood 96, 3847-3856. [PubMed] [Google Scholar]
- 35.Callenbach, P. M. C., Jol-Van der Zijde, C. M., Geerts, A. T., Arts, W. F. M., Van Donselaar, C. A., Peters, A. C. B., Stroink, H., Brouwer, O. F. & van Tol, M. J. D. (2003) Clin. Exp. Immunol. 132, 144-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonnotte, B., Favre, N., Reveneau, S., Micheau, O., Droin, N., Garrido, C., Fontana, A., Chauffert, B., Solary, E. & Martin, F. (1998) Cell Death Differ. 5, 480-487. [DOI] [PubMed] [Google Scholar]
- 37.Maecker, H. L., Yun, Z., Maecker, H. T. & Giaccia, A. J. (2002) Cancer Cell 2, 139-148. [DOI] [PubMed] [Google Scholar]
- 38.Detich, N., Bovenzi, V. & Szyf, M. (2003) J. Biol. Chem. 278, 27586-27592. [DOI] [PubMed] [Google Scholar]
- 39.Bienz, M. & Clevers, H. (2000) Cell 103, 311-320. [DOI] [PubMed] [Google Scholar]
- 40.Yuan, P. X., Huang, L. D., Jiang, Y. M., Gutkind, J. S., Manji, H. K. & Chen, G. (2001) J. Biol. Chem. 276, 31674-31683. [DOI] [PubMed] [Google Scholar]
- 41.Bacon, C. L., Gallagher, H. C., Haughey, J. C. & Regan, C. M. (2002) J. Neurochem. 83, 12-19. [DOI] [PubMed] [Google Scholar]
- 42.Gallagher, H. C., Bacon, C. L., Odumeru, O. A., Gallagher, K. F., Fitzpatrick, T. & Regan, C. M. (2004) Neurotoxicol. Teratol. 26, 73-81. [DOI] [PubMed] [Google Scholar]
- 43.Shin, H. J., Baek, K. H., Jeon, A. H., Kim, S. J., Jang, K. L., Sung, Y. C., Kim, C. M. & Lee, C. W. (2003) Oncogene 22, 3853-3858. [DOI] [PubMed] [Google Scholar]
- 44.Michaelis, M., Michaelis, U. R., Fleming, I., Suhan, T., Cinatl, J., Blaheta, R. A., Hoffmann, K., Kotchetkov, R., Busse, R., Nau, H., et al. (2004) Mol. Pharmacol. 65, 520-527. [DOI] [PubMed] [Google Scholar]
- 45.Jang, E. R., Lim, S. J., Lee, E. S., Jeong, G., Kim, T. Y., Bang, Y. J. & Lee, J. S. (2004) Oncogene 23, 1724-1736. [DOI] [PubMed] [Google Scholar]
- 46.Insinga, A., Monestiroli, S., Ronzoni, S., Gelmetti, V., Marchesi, F., Viale, A., Altucci, L., Nervi, C., Minucci, S. & Pelicci, P. G. (2005) Nat. Med. 11, 71-76. [DOI] [PubMed] [Google Scholar]
- 47.Nebbioso, A., Clarke, N., Voltz, E., Germain, E., Ambrosino, C., Bontempo, P., Alvarez, R., Schiavone, E. M., Ferrara, F., Bresciani, F., et al. (2005) Nat. Med. 11, 77-84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.