Abstract
Background
COVID-19 remains a complex health challenge. We analysed the characteristics and outcomes of COVID-19-related hospitalisations during JN.1 variant dominance.
Methods
Conducted in a hospital serving a socioeconomically deprived population, this study included all adults hospitalised with COVID-19 from 1st November 2023 to 31st August 2024. The primary outcome was in-hospital mortality, analysed in relation to demographic, clinical, and laboratory parameters.
Results
Among 122 individuals (median age 76 years, 58.2% males, median comorbidity index 5), 114/122 (93.4%) had received ≥ 1 SARS-CoV-2 vaccination, with a median of 23 months elapsed since the last dose. Fever (67/122, 54.9%) and dyspnoea (49/122, 40.2%) were common presenting symptoms, with 78/122 (64%) showing CT evidence of SARS-CoV-2 pneumonia; 25/122 (20%) had purely neurological presentations. Treatment included remdesivir (115/122, 94.3%) and/or nirmatrelvir/ritonavir (9/122, 7.4%), sotrovimab (15/122, 12.3%), corticosteroids (61/122, 50.0%), and oxygen supplementation (76/122, 62.3%). Whereas 107/122 (87.7%) were discharged after a median of seven days, in-hospital mortality was 15/122 (12.3%) after a median of 16 days. Baseline factors associated with mortality were neutrophil-lymphocyte ratio > 8, D-dimer ≥ 1800 ng/mL, procalcitonin ≥ 1.0 ng/mL, and albumin < 3.2 g/dL; during admission, nasopharyngeal SARS-CoV-2 antigen positivity persisting for > 12 days, hospitalisation for ≥ 10 days, higher oxygen requirements with the resulting corticosteroid use, and healthcare-associated bacteraemia were associated with increased odds of mortality.
Conclusions
Baseline laboratory parameters and persistent SARS-CoV-2 antigen positivity despite antiviral therapy offer readily available prognostic insights for patients hospitalised with COVID-19. It is imperative to advocate for up-to-date COVID-19 vaccination among older people and other vulnerable groups.
Keywords: COVID-19, SARS-CoV-2, Hospitalisation, Mortality, Remdesivir
Introduction
Despite a notable decline in the burden of COVID-19 and related mortality [1], SARS-CoV-2 continues to pose a threat to human health through successive epidemic waves of varying transmissibility and severity [2]. The Omicron variant emerged in late 2021 and was followed by several subvariants [3, 4]. The JN.1 subvariant was dominant in November 2023-August 2024 when it circulated alongside other subvariants of interest, such as EG.5 and XBB [1]. During November 2023-January 2024, the World Health Organization (WHO) reported increasing COVID-19 cases and associated deaths. These epidemic patterns were also documented in Italy, where numbers peaked on 17–24 December 2023 with 66,300 cases and 466 deaths [1], associated primarily with JN.1, followed by another surge in August 2024 [5].
In addition to viral determinants, factors including age, sex, vaccination status, pre-existing morbidities and coinfections modulate the severity of COVID-19 [6]. Pneumonia remains the leading cause of COVID-19-related hospitalisation [2]. Extrapulmonary manifestations may include cardiovascular, coagulation, renal, gastrointestinal, hepatobiliary, endocrinological, and neurological disorders [7], which, unless recognised as SARS-CoV-2-related, can result in diagnostic delays [8].
The introduction of vaccination represented a turning point in the COVID-19 pandemic [9]. In 2021–2022, Italy adopted strict vaccination policies, making it mandatory for large sectors of the population to receive primary vaccination followed by one additional vaccine dose [10]. Further vaccine doses were recommended for at-risk groups, primarily focusing on older people [10–12]. As of 24th September 2023, 90% of the Italian population over 12 had completed primary vaccination, and 85% of those eligible had received at least one additional vaccine dose [10].
As the characteristics of individuals affected by COVID-19 may evolve with successive epidemic waves and vaccination policies, the aim of this study was to analyse the demographic and clinical profiles of patients hospitalised for COVID-19 in the Infectious Disease (ID) Unit of a tertiary hospital serving an area of socioeconomic deprivation in Italy. The analysis spanned the period November 2023–August 2024. To help improve the management of future surges, we explored factors associated with the outcomes of hospitalisation, which we categorised as discharge from the ID Unit versus in-hospital mortality.
Methods
Study design and setting
This single-centre, observational, retrospective study was based at the ID Unit of the Tor Vergata University Hospital in Rome, Italy, a large tertiary hospital located in the southern suburbs of Rome, serving a population with a high prevalence of socioeconomic deprivation [13].
Study population and investigations
The analysis included all adults (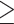 18 years) hospitalised with COVID-19 between 1st November 2023 and 31st August 2024. We recorded the interval between the self-reported onset of symptoms and the admission date. Demographic (age and sex) and clinical data were extracted from the medical records. Laboratory data were retrieved from the hospital’s electronic records. COVID-19 vaccination records were retrieved from the national vaccine registry, including the number of vaccine doses and the date of the last dose. The diagnosis of COVID-19 was based on clinical parameters accompanied by the laboratory-based detection of SARS-CoV-2 antigen in
18 years) hospitalised with COVID-19 between 1st November 2023 and 31st August 2024. We recorded the interval between the self-reported onset of symptoms and the admission date. Demographic (age and sex) and clinical data were extracted from the medical records. Laboratory data were retrieved from the hospital’s electronic records. COVID-19 vaccination records were retrieved from the national vaccine registry, including the number of vaccine doses and the date of the last dose. The diagnosis of COVID-19 was based on clinical parameters accompanied by the laboratory-based detection of SARS-CoV-2 antigen in  1 nasopharyngeal swab (NPS). During admission, the NPS was repeated every five days until negative. Molecular tests for detecting SARS-CoV-2 and other respiratory viruses in NPS were also conducted upon admission, along with routine clinical biochemistry and haematology, immunological profile (CD3+, CD3+ CD4+, CD3+ CD8+, CD19+, CD4:CD8 ratio), d-dimer, procalcitonin, blood cultures, and a chest CT scan. Other baseline investigations and investigations during admission were guided by clinical need.
1 nasopharyngeal swab (NPS). During admission, the NPS was repeated every five days until negative. Molecular tests for detecting SARS-CoV-2 and other respiratory viruses in NPS were also conducted upon admission, along with routine clinical biochemistry and haematology, immunological profile (CD3+, CD3+ CD4+, CD3+ CD8+, CD19+, CD4:CD8 ratio), d-dimer, procalcitonin, blood cultures, and a chest CT scan. Other baseline investigations and investigations during admission were guided by clinical need.
Management of COVID-19
In line with guidelines [14], management of COVID-19 included dexamethasone (6 mg daily for 10 days) in patients who required oxygen supplementation. Oxygen requirements were classified as none, conventional oxygen (i.e., Venturi mask), high-flow nasal cannula, or non-invasive ventilation (HFNC/NIV). Specific treatments included antiviral therapy with remdesivir (200 mg on day 1 followed by 100 mg/day for 3–5 days) or nirmatrelvir/ritonavir (300/100 mg daily for 5 days) and, in selected individuals with immunocompromise, the monoclonal antibody sotrovimab (500 mg as a single intravenous infusion).
Analysis
The primary endpoint was the dichotomous outcome of discharge from the ID Unit or in-hospital mortality. The characteristics of the study population were summarised as categorical variables (expressed as counts and proportions) and continuous variables (expressed as medians with interquartile range [IQR]). We calculated the Charlson Comorbidity Index (CCI), which considers 16 comorbid conditions and age to estimate survival at 10 years, with a higher score associated with higher risk [14]. Calculating severity scores at admission was not part of routine practice. The duration of SARS-CoV-2 positivity was defined as the interval between the first positive and the first negative NPS if a negative test was obtained during hospitalisation. Bacterial and fungal infections detected concomitantly or following the COVID-19 diagnosis were defined as secondary; those with onset > 48 h post-admission were considered healthcare-associated. Variables associated with in-hospital mortality were explored by univariable logistic regression analysis. Where clinically meaningful, continuous variables were transformed to binary, based on laboratory reference ranges, published data, and data distribution in the population. Only variables with clinical or biological justification and binary variables with at least three deaths occurring in the smallest group were modelled to reduce the chance of Type I and II errors. Due to the small number of deaths and the co-linearity of several variables, performing multivariable logistic regression was not considered feasible. The analyses were performed using STATA (Version 18.0 College Station, Texas, USA).
Ethics
The study was approved by the Ethics Committee of Fondazione PTV, University of Rome Tor Vergata (register number 164/20). The requirement for informed consent was waived by the Ethics Committee, considering the retrospective, observational nature of the study, provided the electronic database was anonymised after data collection. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Results
Characteristics of the study population at the time of hospital admission
The study population comprised 122 consecutive individuals admitted to the ID Unit with COVID-19 between 1st November 2023 and 31st August 2024. Their characteristics are summarised in Table 1. The median age was 76 years, with males comprising a slight majority. Most participants had previously received 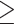 1 and usually
1 and usually 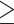 3 doses of a COVID-19 vaccine, whereas 7/122 (5.7%) were unvaccinated. The median time from the last vaccine dose to the date of admission was 23 months; only 12/122 (9.8%) had received a vaccine dose in the previous 12 months.
3 doses of a COVID-19 vaccine, whereas 7/122 (5.7%) were unvaccinated. The median time from the last vaccine dose to the date of admission was 23 months; only 12/122 (9.8%) had received a vaccine dose in the previous 12 months.
Table 1.
Characteristics of the study population at admission, overall and by outcome
| Characteristics | Total (n = 122) |
Outcome | ||
|---|---|---|---|---|
| Discharge (n = 107) | Mortality (n = 15) | |||
| Age, median years (IQR) | 76 (69–84) | 76 (67–84) | 78 (72–84) | |
| Male sex, n (%) | 71 (58.2) | 63 (58.9) | 8 (53.3) | |
|
COVID-19 vaccine, n (%) |
None | 7 (5.7) | 7 (6.5) | 0 (0) |
| 1 dose | 1 (0.8) | 0 (0) | 1 (6.7) | |
| 2 doses | 8 (6.6) | 8 (7.5) | 0 (0) | |
| 3 doses | 67 (54.9) | 57 (53.3) | 10 (66.7) | |
| > 3 doses | 39 (32.0) | 35 (32.7) | 4 (26.7) | |
| Time since last vaccination, median months (IQR) | 23.3 (17.7–25.4) | 23.3 (17.6–25.6) | 23.3 (21.9–24.2) | |
| Comorbidity index, median (IQR) | 5 (4–6) | 5 (4–6) | 5 (4–7) | |
|
Comorbidities, n (%) |
Cardiovascular disease | 91 (74.6) | 82 (76.6) | 9 (60.0) |
| Neurovascular disease | 34 (27.9) | 30 (28.0) | 4 (28.6) | |
| Immunodeficiencya | 31 (25.4) | 26 (24.3) | 5 (33.3) | |
| Pulmonary diseaseb | 24 (19.7) | 19 (17.8) | 5 (33.3) | |
| Diabetes (type II) | 30 (24.6) | 28 (26.2) | 2 (13.3) | |
| Chronic renal disease | 18 (14.8) | 18 (16.8) | 0 (0) | |
| Obesity | 8 (6.6) | 6 (5.6) | 2 (13.3) | |
| Symptoms, n (%) | Fever | 67 (54.9) | 61 (57.0) | 6 (40.0) |
| Dyspnoea | 49 (40.2) | 40 (37.4) | 9 (60.0) | |
| Neurologicalc | 51 (41.8) | 45 (42.1) | 6 (40.0) | |
| Time since symptoms onset, median days (IQR) | 2.5 (1–6) | 2 (1–6) | 3 (1–7) | |
| SARS-CoV-2 pneumonia, n (%) | 78 (63.9) | 65 (60.7) | 13 (86.7) | |
| Secondary infectiond, n (%) | Any | 50 (41.0) | 45 (42.1) | 5 (33.3) |
| Pneumonia | 24 (19.7) | 22 (20.6) | 2 (13.3) | |
| UTI | 15 (12.3) | 15 (14.0) | 0 (0) | |
| Bacteraemia | 8 (6.6) | 8 (7.5) | 0 (0) | |
| Othere | 3 (2.5) | 3 (2.8) | 0 (0) | |
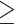 1 microorganism identified 1 microorganism identified |
31 (25.4) | 29 (27.1) | 2 (13.3) | |
| Respiratory virusf, n (%)f | 4 (3.3) | 2 (1.9) | 2 (13.3) | |
| Haemoglobin, median g/dL (IQR) | 11.4 (9.9–12.8) | 11.5 (10.0-12.9) | 10.8(9.3–12.5) | |
| MCH, median pg (IQR) | 33 (30–34) | 33 (30–34) | 31 (29–34) | |
| Platelets, median cells x103/µL (IQR) | 184 (127–237) | 182 (132–227) | 186 (113–288) | |
| WBC, median cells × 109/L (IQR) | 6.5 (4.8–9.5) | 6.5 (4.8–9.4) | 9.1 (4.1–11.7) | |
| Neutrophil count, median cells × 109/L (IQR) | 5.2 (3.2-8.0) | 4.9 (3.1–7.8) | 7.6 (4.8–10.3) | |
| Lymphocyte count, median cells × 109/L (IQR) | 0.9 (0.5–1.3) | 0.9 (0.6–1.4) | 0.6 (0.3–1.1) | |
| Neutrophil-lymphocyte ratio, median (IQR) | 5.7 (3.1–9.6) | 4.8 (2.9–8.8) | 12.9 (6.6–15.2) | |
| Monocyte count, median cells × 109/L (IQR) | 0.5 (0.3–0.7) | 0.5 (0.3–0.7) | 0.4 (0.2–0.6) | |
| CD3 + median cells/µL (IQR) | 804 (463–1041) | 812 (468–1100) | 485 (257–766) | |
| CD3 + CD4 + median cells/µL (IQR) | 437 (252–624) | 484 (257–630) | 283 (162–435) | |
| CD3 + CD8 + median cells/µL (IQR) | 244 (153–395) | 249 (159–424) | 201 (93–281) | |
| CD19 + median cells/µL (IQR) | 59 (32–113) | 58 (32–116) | 59 (34–90) | |
| CD4:CD8 ratio | 1.9 (1.1–3.1) | 2.04 (1.1–3.1) | 1.4 (1.1–2.1) | |
| Creatinine, median mg/dL (IQR) | 0.99 (0.75–1.37) | 1.0 (0.73–1.37) | 0.96 (0.8–1.67) | |
| AST, median U/L (IQR) | 27 (20–42) | 26 (20–40) | 35 (24–56) | |
| Albumin, median g/dl (IQR) | 3.4 (3.0-3.8) | 3.4 (3.1–3.8) | 2.9 (2.6–3.7) | |
| Blood glucose, median mg/dL (IQR) | 105 (85–132) | 104 (84–129) | 127 (98–152) | |
| Ferritin, median ng/ml (IQR) | 401 (165–956) | 401 (168–805) | 446 (137–1399) | |
| CRP, median mg/mL (IQR) | 65.8 (31.6–140) | 64.9 (31.6–129) | 137 (25.6-185.1) | |
| LDH, median U/L (IQR) | 217 (185–282) | 216 (180–277) | 252 (189–302) | |
| D-dimer, median ng/mL (IQR) | 1305 (733–2451) | 1199 (636–2177) | 3334 (1755–4363) | |
| BNP, median pg/mL (IQR) | 191 (88–394) | 184 (90–375) | 280 (70–488) | |
| Procalcitonin, median ng/mL (IQR) | 0.14 (0.05–0.71) | 0.13 (0.05–0.55) | 1.24 (0.11–2.84) | |
aIncluding haematological (n = 13) and solid (n = 4) neoplasms, history of cardiac or renal transplantation (n = 2), immunosuppressive treatment for rheumatoid arthritis (n = 2), and HIV infection (n = 2). bIncluding chronic obstructive pulmonary disease, emphysema, and obstructive sleep apnoea syndrome. cIncluding altered mental status, syncope, seizures, cephalgia, diplopia, hemiparesis, and dizziness. dNumber of patients with suspected infection with onset within 48 h of admission. eComprising two cases of endocarditis (methicillin-sensitive and methicillin-resistant S. aureus, respectively) and one cholecystitis (no microorganism identified). fComprising three cases with rhinovirus and one case with both influenza H1N1 and parainfluenza type 4, all detected within 48 h of admission. Abbreviations: AST, aspartate aminotransferase; BNP, B-type natriuretic peptide; CRP, C-reactive protein; IQR, interquartile range; LDH, lactate dehydrogenase; MCH, mean corpuscular haemoglobin; UTI, Urinary tract infection; WBC, white blood cells
There was a high prevalence of comorbidities, with a median comorbidity index of 5. Prevalent comorbidities comprised cardiovascular disease (predominantly hypertension), neurovascular disease, chronic pulmonary disease (including chronic obstructive pulmonary disease, emphysema, and obstructive sleep apnoea syndrome), and type II diabetes (Table 1). Overall, 31/122 (25.4%) had immunocompromise as a result of haematological and solid neoplasms, long-standing (approximately 10 years) immunosuppressive therapy following cardiac or renal transplantation, treatment for rheumatoid arthritis with methotrexate, or HIV infection. The two individuals with HIV infection were receiving virologically suppressive antiretroviral therapy (plasma HIV-1 RNA < 100 copies/mL) but had a low CD4 count recorded before admission (191 and 17 cells/mm3, respectively).
Presenting symptoms most frequently included fever and dyspnoea (Table 1). However, 51/122 (41.8%) had neurological symptoms, especially altered mental status (33/122, 27%) or seizures (4/122, 3.3%), and less frequently cephalgia, diplopia, hemiparesis and dizziness. Neurovascular or cardiovascular syncope was the presenting symptom in 15/122 (12.3%). In 8/122 (6.5%) the presentation was with generalised pain or having suffered a fall. The chest CT scan showed changes consistent with SARS-CoV-2 pneumonia in 78/122 (63.9%) patients on admission. Of the 51 patients with neurological symptoms, 25 lacked CT scan evidence of pneumonia, resulting in 25/122 (20.5%) individuals with purely neurological presentations. Laboratory test results are summarised in Table 1.
The course of hospital stay
Overall, 76/122 (62.3%) participants had a SARS-CoV-2 antigen-negative NPS documented a median of five (5–12) days after admission. Of the remaining 46, 39 were discharged with a positive NPS, whereas seven died while still testing positive for SARS-CoV-2. Of the 104/122 (85.2%) individuals who underwent NPS molecular testing, 93/104 (89.4%) had SARS-CoV-2 RNA detected, whereas 11/104 (10.5%), all sampled 3–5 days after the initial SARS-CoV-2 antigen positive NPS, tested SARS-CoV-2 RNA negative. Most participants (76/122, 62.3%) required oxygen supplementation, with the use of a Venturi mask in 49/122 (40.2%) and HFNC/NIV in 27/122 (22.1%). A total of 61/122 (50.0%) individuals received corticosteroids (usually dexamethasone), including 59 who needed oxygen supplementation and two who did not but were already on systemic steroids at admission. Seven individuals with minimal oxygen supplementation did not receive corticosteroids. Antiviral therapy comprised remdesivir in 115/122 (94.3%) and nirmatrelvir/ritonavir in 9/122 (7.4%); two patients, one with non-Hodgkin lymphoma and one with prostatic cancer, received sequential therapy with both antivirals. In addition, 15/122 (12.3%) patients, all with immunocompromise, received sotrovimab.
Other infections
Four individuals (3.3%) had concomitant molecular detection of SARS-CoV-2 and either rhinoviruses (n = 3) or both influenza H1N1 and parainfluenza type 4 (n = 1); all detections were within 48 h of admission (Table 1). Either at or during admission, 74/122 (60.6%) individuals had 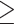 1 suspected secondary bacterial infection based on clinical and, where appropriate, imaging findings, for which they received antimicrobial therapy. Among these, 52/122 (46.6%) had
1 suspected secondary bacterial infection based on clinical and, where appropriate, imaging findings, for which they received antimicrobial therapy. Among these, 52/122 (46.6%) had 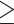 1 microorganism identified, including 21 with bacterial pneumonia (primarily S. pneumoniae, P. aeruginosa, and H. influenzae), 24 with urinary tract infection (UTI) (primarily E. coli, E. faecalis, and K. pneumoniae), and 13 with bacteraemia (primarily coagulase-negative staphylococcus, S. aureus, and K. pneumoniae). Whereas most secondary bacterial infections were diagnosed concomitantly or soon after the COVID-19 diagnosis (Table 1), 24/74 (32.4%) individuals with a suspected infection and 21/52 (48.1%) of those with ≥1 microorganism identified were considered to have acquired the infection while in hospital based on onset >48 h post-admission (median 8 days [IQR 6–16]) (Table 2).
1 microorganism identified, including 21 with bacterial pneumonia (primarily S. pneumoniae, P. aeruginosa, and H. influenzae), 24 with urinary tract infection (UTI) (primarily E. coli, E. faecalis, and K. pneumoniae), and 13 with bacteraemia (primarily coagulase-negative staphylococcus, S. aureus, and K. pneumoniae). Whereas most secondary bacterial infections were diagnosed concomitantly or soon after the COVID-19 diagnosis (Table 1), 24/74 (32.4%) individuals with a suspected infection and 21/52 (48.1%) of those with ≥1 microorganism identified were considered to have acquired the infection while in hospital based on onset >48 h post-admission (median 8 days [IQR 6–16]) (Table 2).
Table 2.
Events during hospitalisation overall and by outcome
| Events | Total (n = 122) |
Outcome | |
|---|---|---|---|
| Discharge (n = 107) | Mortality (n = 15) | ||
| Secondary infectiona, n (%) | |||
| Any | 24 (19.7) | 17 (15.9) | 7 (46.7) |
| Pneumonia | 8 (6.6) | 6 (5.6) | 2 (13.3) |
| UTI | 9 (7.4) | 7 (6.5) | 2 (13.3) |
| Bacteraemia | 7 (5.7) | 3 (2.8) | 4 (26.7) |
| Otherb | 6 (4.9) | 4 (3.7) | 2 (13.3) |
| ≥ 1 microorganism identified | 21 (17.2) | 15 (14.0) | 6 (40) |
| Oxygen, n (%) | |||
| None | 46 (37.7) | 45 (42.1) | 1 (6.7) |
| Venturi mask | 49 (40.2) | 44 (41.1) | 5 (33.3) |
| HFNC/NIV | 27 (22.1) | 18 (16.8) | 9 (60.0) |
| Therapies, n (%) | |||
| Antiviral therapy (any) | 122 (100.0) | 107 (100.0) | 15 (100.0) |
| Remdesivir | 115 (94.3) | 100 (93.4) | 15 (100.0) |
| Nirmatrelvir/Ritonavir | 9 (7.4) | 9 (8.4) | 0 (0.0) |
| Corticosteroids | 61 (50.0) | 47 (43.9) | 14 (93.3) |
| Sotrovimab | 15 (12.3) | 14 (13.1) | 1 (6.7) |
| Time to negative NPSc, median days (IQR) | 5 (3–11) | 5 (3–8) | 12.5 (8–15) |
| Length of hospital stay, median days (IQR) | 7 (4–15) | 7 (4–12) | 16 (3–26) |
aNumber of patients with onset of infection > 48 h of admission. bComprising 3 cases of C. difficile colitis, 2 cases of candidemia (C. albicans and C. parapsilosis), and one wound infection with vancomycin-resistant enterococcus. cInterval between the first positive and the first negative NPS if a negative test was obtained during hospitalisation. Abbreviations: HFNC, high flow nasal cannula; IQR, interquartile range; NIV, non-invasive ventilation; NPS, nasopharyngeal swab; UTI, urinary tract infection
Outcomes
No patient underwent admission to intensive care. A total of 107/122 (87.7%) individuals were discharged from the ID Unit after a median of seven days (IQR 4–15), whereas 15/122 (12.3%) died while still in the Unit after a median of 16 (IQR 3–26) days of hospitalisation The cause of death was attributed to respiratory (n = 12), cardiovascular (n = 3) or gastrointestinal (n = 1) complications, sepsis (n = 3), and progression of underlying malignancy (n = 2).
Factors associated with in-hospital mortality
Results of the univariable analysis exploring factors associated with in-hospital mortality are shown in Table 3. We did not see marked differences according to age and sex, number of COVID-19 vaccine doses, comorbidities, presenting symptoms, or the occurrence of secondary infections within 48 h of admission. Baseline factors associated with increased odds of in-hospital mortality included N/L ratio > 8 (OR 5.37), D-dimer levels ≥ 1800 ng/mL (OR 4.80), procalcitonin levels ≥ 1.0 ng/mL (OR 3.56), and albumin levels ≤ 3.2 g/dL (OR 3.5). During admission, factors associated with increased odds of mortality were duration of hospital stay 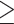 10 days (OR 3.36 vs. <10 days) and time to first negative NPS >12 days (OR 3.38 vs. ≤12 days). Other associated parameters included requiring HFNC/NIV (OR 2.72), receiving corticosteroid therapy (OR 9.67), and the occurrence of healthcare-associated bacteraemia (OR 12.60).
10 days (OR 3.36 vs. <10 days) and time to first negative NPS >12 days (OR 3.38 vs. ≤12 days). Other associated parameters included requiring HFNC/NIV (OR 2.72), receiving corticosteroid therapy (OR 9.67), and the occurrence of healthcare-associated bacteraemia (OR 12.60).
Table 3.
Univariable analysis of factors associated with in-hospital mortality
| Variable | OR (95% CI) | P |
|---|---|---|
| Characteristics at admission | ||
| Age | ||
| Per 1 year older | 1.02 (0.97–1.07) | 0.38 |
| Sex | ||
| Female | REF | |
| Male | 0.79 (0.26–2.36) | 0.68 |
| COVID-19 vaccine | ||
| 1–3 doses (n = 76) | REF | |
| > 3 doses (n = 39) | 0.67 (0.20–2.27) | 0.52 |
| Interval last vaccine dose to admission | ||
| Per 1 month higher | 1.01 (0.93–1.1) | 0.68 |
| Comorbidity Index | ||
| Per 1 point higher | 1.22 (0.91–1.65) | 0.17 |
| Comorbidities (yes vs. no) | ||
| Cardiovascular disease | 0.45 (0.14–1.40) | 0.17 |
| Neurovascular disease | 0.93 (0.27–3.16) | 0.91 |
| Immunodeficiency | 1.55 (0.48–4.97) | 0.45 |
| Pulmonary disease | 2.31 (0.70–7.55) | 0.16 |
| Symptoms (yes vs. no) | ||
| Fever | 0.50 (0.16–1.51) | 0.22 |
| Dyspnoea | 2.51 (0.83–7.58) | 0.10 |
| Neurological | 0.91 (0.30–2.76) | 0.88 |
| Interval symptom onset to admission | ||
| Per 1 day longer | 1.02 (0.95–1.1) | 0.53 |
| SARS-CoV-2 pneumonia (yes vs. no) | 4.2 (0.90–19.6) | 0.06 |
Secondary infection 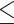 48 hours (yes vs. no) 48 hours (yes vs. no) | ||
| Any | 0.66 (0.21–2.05) | 0.47 |
| Haemoglobin (g/dL) | ||
| ≤ 12 (n = 75) | REF | |
| > 12 (n = 47) | 0.77 (0.24–2.42) | 0.66 |
| MCH (pg) | ||
| ≤ 33 (n = 57) | REF | |
| > 33 (n = 65) | 0.39 (0.12–1.22) | 0.10 |
| Platelets (cells x103/µL) | ||
| ≤ 150 (n = 41) | REF | |
| > 150 (n = 81) | 0.72 (0.24–2.21) | 0.57 |
| WBC (cells × 109/L) | ||
| ≤ 10.0 (n = 95) | REF | |
| > 10.0 (n = 27) | 1.93 (0.59–6.23) | 0.27 |
| Neutrophil count (× 109/L) | ||
| ≤ 7 (n = 76) | REF | |
| > 7.0 (n = 46) | 0.6 (0.41–0.87) | 0.007 |
| Lymphocyte count (× 109/L) | ||
| ≤ 1.0 (n = 65) | REF | |
| > 1.0 (n = 57) | 0.52 (0.16–1.65) | 0.27 |
| Neutrophil-lymphocyte ratio | ||
| ≤ 8 (n = 83) | REF | |
| > 8 (n = 39) | 5.37 (1.69–17.1) | < 0.01 |
| Monocyte count (× 109/L) | ||
| ≤ 500 (n = 64) | REF | |
| > 500 (n = 58) | 0.96 (0.32–2.83) | 0.94 |
| CD3+ (cells/µL) | ||
| ≤ 500 (n = 25) | REF | |
| > 500 n (= 97) | 0.45 (0.14–1.49) | 0.19 |
| CD3 + CD4+ (cells/µL) | ||
| ≤ 300 (n = 27) | REF | |
| > 300 (n = 95) | 0.51 (0.16–1.67) | 0.27 |
| CD3 + CD8+ (cells/µL) | ||
| ≤ 300 (n = 49) | REF | |
| > 300 (n = 73) | 0.54 (0.18–1.61) | 0.27 |
| CD19+ (cells/µL) | ||
| ≤ 60 (n = 41) | REF | |
| > 60 (n = 81) | 1.01 (0.32–3.18) | 0.98 |
| CD4:CD8 ratio | ||
| ≤ 2 (n = 42) | REF | |
| > 2 (n = 80) | 0.76 (0.25–2.30) | 0.62 |
| Creatinine (mg/dL) | ||
| ≤ 1.2 (n = 55) | REF | |
| > 1.2 (n = 31) | 1.21 (0.40–3.65) | 0.73 |
| AST (U/L) | ||
| ≤ 35 (n = 82) | REF | |
| > 35 (n = 40) | 1.96 (0.65–5.86) | 0.22 |
| Albumin (g/dl) | ||
| > 3.2 (n = 81) | REF | |
| ≤ 3.2 (n = 41) | 3.51 (1.15–10.69) | 0.02 |
| Blood glucose (mg/dL) | ||
| ≤ 100 (n = 55) | REF | |
| > 100 (n = 67) | 1.75 (0.56–5.47) | 0.33 |
| Ferritin (ng/ml) | ||
| ≤ 275 (n = 30) | REF | |
| > 275 (n = 92) | 0.88 (0.25-3.00) | 0.84 |
| CRP (mg/L) | ||
| ≤ 50 (n = 45) | REF | |
| > 50 (n = 77) | 1.70 (0.50–5.72) | 0.38 |
| LDH (U/L) | ||
| ≤ 220 (n = 63) | REF | |
| > 220 (n = 59) | 1.71 (0.56–5.14) | 0.33 |
| D-dimer (ng/mL) | ||
| < 1800 (n = 72) | REF | |
| > 1800 (n = 50) | 4.80 (1.42–16.1) | 0.01 |
| BNP (pg/mL) | ||
| ≤ 200 (n = 43) | REF | |
| > 200 (n = 79) | 1.57 (0.47–5.29) | 0.46 |
| Procalcitonin (ng/mL) | ||
| ≤ 1.0 (n = 88) | REF | |
| > 1.0 (n = 34) | 3.56 (1.17–10.76) | 0.02 |
|
Events during hospitalisation Secondary infection > 48 h (yes vs. no) | ||
| Any | 4.63 (1.48–14.47) | < 0.01 |
| Bacteraemia | 12.60 (2.49–63.75) | < 0.01 |
| Oxygen supplementation | ||
| None/venturi mask | REF | |
| HFNC/NIV | 2.72 (1.53–4.84) | < 0.01 |
| Therapy (yes vs. no) | ||
| Corticosteroids | 9.67 (2.07–45.03) | < 0.01 |
| Time to negative NPS | ||
| ≤ 12 days (n = 63) | REF | |
| > 12 days (n = 59) | 3.38 (1.01–11.29) | 0.04 |
| Length of hospital stay | ||
| < 10 days (n = 80) | REF | |
| ≥ 10 days (n = 42) | 3.36 (1.10-10.22) | 0.03 |
Abbreviations: AST, aspartate aminotransferase; BNP, B-type natriuretic peptide; CRP, C-reactive protein; HFNC, high flow nasal cannula; IQR, interquartile range; LDH, lactate dehydrogenase; MCH, mean corpuscular haemoglobin; NIV, non-invasive ventilation; NPS, nasopharyngeal swab; UTI, urinary tract infection; WBC, white blood cells
Discussion
We analysed the clinical presentation of COVID-19 during the 2023–2024 JN-1-related surge in admissions to the ID Unit of a large hospital serving an area with a high prevalence of socioeconomic deprivation. The population requiring admission had a median age of 76 years, included almost equal numbers of men and women, and commonly had comorbidities. Although the majority (∼87%) had received COVID-19 vaccination, a minority (∼10%) had received a vaccine dose in the previous 12 months. At admission, most (∼64%) had CT findings consistent with SARS-CoV-2 pneumonia; however, purely neurological presentations without pneumonia were seen in nearly 20%. Despite standard-of-care therapy and with no patient progressing to intensive care [15], (one in eight) individuals died during the admission. Several factors documented at admission were observed to predict the crude odds of mortality, including higher N/L ratio, higher d-dimer and procalcitonin levels, and lower albumin levels. During the admission, prolonged SARS-CoV-2 positivity (based on antigen testing of NPS), prolonged hospital stay, higher oxygen requirements with the resulting use of corticosteroids, and occurrence of healthcare-associated bacteraemia were associated with increased odds of mortality.
The characteristics of patients hospitalised with COVID-19 have evolved through successive epidemic waves. During the early stages of the pandemic, patients in our Unit had a median age of 62 years [16], and pneumonia was the most common presentation (> 94% of cases). Consistent with recent observations [17], the 2023–2024 population was considerably older. They less frequently presented with pneumonia, which may reflect the clinical features of COVID-19 in the post-vaccination era and improved awareness of other presenting features. Notably, 42% of patients presented with neurological syndromes, and almost one in two of these had no respiratory symptoms. A cohort study similarly found that one in seven patients with COVID-19 presented without respiratory symptoms [8], underscoring the importance of considering extrapulmonary symptoms in managing COVID-19.
Although we had no admissions to intensive care (vs. 17% in the early stages of the pandemic [16]), the in-hospital mortality was nearly 13% and primarily attributed to severe respiratory disease. Following guidelines, most participants received antiviral therapy, typically with remdesivir rather than nirmatrelvir-ritonavir. We did not have access to other therapeutic agents such as molnupiravir, baricitinib, anakinra, tocilizumab, or vilobelimab. Recent analyses suggest that nirmatrelvir-ritonavir is more effective than remdesivir in reducing mortality risk, intensive care admission, and the need for ventilatory support in patients hospitalised with COVID-19 [18]. We added sotrovimab in a subset with immunocompromise. Although sotrovimab is no longer recommended, a few studies suggest it may retain some benefit [19, 20]. More broadly, combining different antivirals or antivirals plus immunotherapeutics has been proposed for individuals with or at risk of severe COVID-19 [21–25]. However, the evidence for combination therapies is inconsistent, and practice varies [18, 26, 27].
Identifying prognostic factors that can be measured at admission can help guide therapeutic interventions, and this has been a research focus. Several severity scores have been considered to stratify patients with COVID-19 and predict outcomes. Some, like the NEWS2 (National Early Warning Score 2), use general physiological parameters (respiratory rate, oxygen saturation, temperature, etc.) [28], whereas others are COVID-specific and also incorporate laboratory parameters, like the 4 C Mortality Score (urea, CRP) [29] or the COVID-GRAM Risk Score (N/L ratio, LDH, direct bilirubin) [30]. We did not use scores in our routine practice. Here, we found that N/L ratio > 8, D-dimer ≥ 1800 ng/mL, procalcitonin ≥ 1.0 ng/mL, and albumin ≤ 3.2 g/dL at admission were associated with increased crude odds of mortality. These findings are consistent with the proposed prognostic role of these readily available laboratory biomarkers [31].
The N/L ratio reflects the innate response to systemic inflammation, injury and stress characterised by lymphocytopenia and neutrophilia. Cut-off values have not been firmly determined, but mean N/L values ranged between 6 and 10 in studies that related the N/L ratio to COVID-19 severity and mortality [32]. This is consistent with our proposed cut-off of > 8. D-dimer has been previously identified as an early indicator of altered coagulation and a predictor of mortality in COVID-19. In a study of 180 patients, the mean admission D-dimer was 1067 ng/ml among surviving patients vs. 3208 ng/ml among patients who died, and a level of 1500 ng/ml was suggested as the threshold for predicting COVID-19-related mortality [33]. Applying a cut-off of 1800 ng/mL was associated with almost 5-fold higher odds of mortality in our study, which increased to 7-fold if lowering the cut-off to 1500 ng/mL (data not shown). Further studies are needed to refine these thresholds.
Procalcitonin is another early marker of systemic inflammatory responses [34]. Whilst published data have been discordant, a systematic analysis of 10 studies with 7716 patients concluded that individuals with COVID-19 and elevated procalcitonin on admission had a 77% increased risk of mortality [35]. The magnitude of the effect was reduced upon adjusting for diabetes, but we could not assess the interaction due to small numbers. Hypoalbuminemia has been previously linked to disease severity and mortality among patients with COVID-19 [36, 37]. In one study, the odds of mortality increased nearly 4-fold among patients with albumin levels < 3.5 g/dl [37], which is consistent with our observations.
The range of proposed prognostic biomarkers in COVID-19 is broad; we did not find that baseline haemoglobin, platelets, creatinine, AST, blood glucose, CRP, LDH, ferritin, BNP, or CD3+, CD4+, CD8 + and CD19 + cells differed substantially by outcome in this small cohort. Two parameters appeared to provide a potentially helpful prognostic indicator during the hospital admission. The first was the ongoing NPS SARS-CoV-2 positivity, which may serve as an indicator for intensified or modified antiviral therapy. The second parameter was admission prolonging for 10 days or more. The two parameters were found to be related in a study from China, which showed that prolonged virus shedding was predictive of prolonged hospital stay in patients with COVID-19 [38]. Since dexamethasone is recommended for individuals with COVID-19 who require oxygen supplementation [14], indicative of greater disease severity, it was anticipated that both the need for HFNC/NIV and corticosteroid therapy would be associated with increased mortality odds.
Concomitant detection of other respiratory viruses was rare (∼3%). Secondary bacterial and, rarely, fungal infections, mainly pneumonia, UTI and bacteraemia, were diagnosed in ∼47% of patients, increasing to ∼61% when including individuals with a suspected infection but no identified microorganism. When examining infections with onset within 48 h of admission, bacterial pneumonia was diagnosed in ∼12% of patients. This is at the higher end of the spectrum reported in earlier studies [39] but consistent with recent winter observations [40] and findings among older individuals with comorbidities [41]. The spectrum of microorganisms also aligns with previous reports [39–42]. A 2016 national point prevalence survey indicated that ∼8% of patients in Italian acute care hospitals had at least one healthcare-associated infection on the survey day [43]. The higher proportion of patients (∼17%) with healthcare-associated infections we observed may indicate limited progress in systematic prevention measures but also likely reflects increased COVID-19-related vulnerability due to prolonged hospitalisation, frequent invasive procedures, and underlying comorbidities [44]. We did not find that the occurrence of bacterial pneumonia differed substantially by outcome; however, as reported by others [45], there was a higher incidence of healthcare-associated bacteraemia among people who died.
In our analysis, only 12 patients had received a COVID-19 vaccine dose in the previous 12 months. Yet, the consensus is that updated vaccinations are particularly beneficial for individuals aged ≥ 60 years and immunocompromised patients [46, 47]. In Italy, annual vaccination is recommended for at-risk groups and those aged 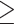 60 [10]. However, vaccination uptake in Italy overall and in the Lazio region, including Rome, has declined for each subsequent dose [10]. As of September 2023, proportions of the Lazio population that had received a first, second and third additional dose after completing primary vaccination were 91%, 19% and 1%, respectively, among people aged 60–69, 93%, 31% and 2% among those aged 70–79 years, and 91%, 44% and 5% among those aged ≥80 years; similar proportions were observed in other Central and Northern Italian regions [10]. Given substantial evidence that vaccine effectiveness against severe COVID-19 outcomes wanes over time [48–50], enhanced public health interventions must emphasise the importance of regular vaccinations for older people and other vulnerable populations.
60 [10]. However, vaccination uptake in Italy overall and in the Lazio region, including Rome, has declined for each subsequent dose [10]. As of September 2023, proportions of the Lazio population that had received a first, second and third additional dose after completing primary vaccination were 91%, 19% and 1%, respectively, among people aged 60–69, 93%, 31% and 2% among those aged 70–79 years, and 91%, 44% and 5% among those aged ≥80 years; similar proportions were observed in other Central and Northern Italian regions [10]. Given substantial evidence that vaccine effectiveness against severe COVID-19 outcomes wanes over time [48–50], enhanced public health interventions must emphasise the importance of regular vaccinations for older people and other vulnerable populations.
This study has limitations. It is a single-centre study with a small sample size, which limits the generalisability of the findings and our ability to explore additional associations or perform adjusted analyses. Nonetheless, the data point to several easily measurable prognostic indicators at admission and during admission that may help guide management strategies in future epidemic surges. Studies should be planned to explore the clinical utility of incorporating these additional indicators in patient stratification scores.
Acknowledgements
We thank the medical and nursing staff of the Infectious Disease Unit of Policlinico Tor Vergata, Rome, Italy for their support.
Abbreviations
- AST
Aspartate aminotransferase
- BNP
B-type natriuretic peptide
- CCI
Charlson comorbidity index
- CRP
C-reactive protein
- CT
Computerised tomography
- HFNC
High-flow nasal cannula
- HIV
Human immunodeficiency virus
- ID
Infectious diseases
- IQR
Interquartile range
- LDH
Lactate dehydrogenase
- MCH
Mean corpuscular haemoglobin
- NEWS2
National early warning score 2
- N/L
Neutrophil-lymphocyte ratio
- NIV
Non-invasive ventilation
- NPS
Nasopharyngeal swab
- STROBE
Strengthening the reporting of observational studies in epidemiology
- UTI
Urinary tract infection
- WBC
White blood cells
- WHO
World health organization
Author contributions
DZ contributed to data collection, performed statistical analysis and wrote the manuscript. AÇ contributed to data collection and to the first draft of the manuscript. CJ supervised the statistical analysis. MI designed the study, supervised data collection and contributed to the analysis and writing of the manuscript. AMG designed the study, supervised data collection and analysis, and wrote the manuscript. LS, LF, GDS, ET and VM critically revised the manuscript. All authors approved the final version of the manuscript.
Funding
DZ and AC were funded through a speciality training fellowship.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Fondazione PTV, University of Rome Tor Vergata (register number 164/20). The Ethics Committee waived the requirement for informed consent based on the retrospective, observational nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. WHO-COVID19 dashboard. Available at: https://data.who.int/dashboards/covid19 (accessed Dec 2024).
- 2.Roser LP, Samanapally H, Ali T, et al. Different clinical characteristics and outcomes of adult hhospitalisedSARS-CoV-2 pneumonia patients complicated by cardiovascular events during the first, delta and omicron waves of COVID-19. Front Epidemiol. 2024;4:1342917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC). COVID Data Tracker. Available at: https://covid.cdc.gov/covid-data-tracker/#variant-summary (accessed Dec 2024).
- 4.Lewnard JA, Mahale P, Malden D, et al. Immune escape and attenuated severity associated with the SARS-CoV-2 BA.2.86/JN.1 lineage. Nat Commun. 2024;15:8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Istituto Superiore di Sanità. COVID-19 integrated surveillance: key national data. Available at: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-integrated-surveillance-data (accessed Dec 2024).
- 6.Zhang JJ, Dong X, Liu GH, Gao YD. Risk and protective factors for COVID-19 morbidity, severity, and mortality. Clin Rev Allergy Immunol. 2023;64:90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JO, Cho HK, Jeon CH, et al. Characteristics and biomarkers associated with mortality in COVID-19 patients presenting to the emergency department. Epidemiol Infect. 2024;152:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Citarella BW, Kartsonaki C, Ibáñez-Prada ED. Characteristics and outcomes of COVID-19 patients admitted to hospital with and without respiratory symptoms. Heliyon. 2024;10:e29591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauring AS, Tenforde MW, Chappell JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376:e069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ministry of Health. COVID-19. Available at: https://www.salute.gov.it/portale/nuovocoronavirus/archivioNormativaNuovoCoronavirus.jsp and https://www.salute.gov.it/reportVacciniAntiCovid/ (accessed Dec 2024).
- 11.Istituto Superiore di Sanità. Vaccines & immunisations. Available at: https://www.epicentro.iss.it/en/vaccines/ (accessed Dec 2024).
- 12.Fabiani M, Mateo-Urdiales A, Sacco C, et al. Effectiveness against severe COVID-19 of a seasonal booster dose of bivalent (original/Omicron BA.4–5) mRNA vaccines in persons aged ≥ 60 years: estimates over calendar time and by time since administration during prevalent circulation of different Omicron subvariants, Italy, 2022–2023. Vaccine. 2024;42:126026. [DOI] [PubMed] [Google Scholar]
- 13.Lelo K, Monni S, Tomassi F. Dec Roma, tra centro e periferie: Come incidono le dinamiche urbanistiche sulle disuguaglianze socio-economiche. Available at: https://www.academia.edu/28425463/Roma_tra_centro_e_periferie_come_incidono_le_dinamiche_urbanistiche_sulle_disuguaglianze_socio_economiche (accessed 2024).
- 14.Gulick RM, Pau AK, Daar E, et al. National Institutes of Health COVID-19 treatment guidelines panel: perspectives and lessons learned. Ann Intern Med. 2024;177:1547–57. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 16.Iannetta M, Buccisano F, Fraboni D, et al. Baseline T-lymphocyte subset absolute counts can predict both outcome and severity in SARS-CoV-2 infected patients: a single center study. Sci Rep. 2021;11:12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Y, Choi T, Al-Aly Z. Mortality in patients hospitalised for COVID-19 vs influenza in fall-winter 2023–2024. JAMA. 2024;331:1963–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi MH, Wan EYF, Wong ICK, et al. Comparative effectiveness of combination therapy with nirmatrelvir-ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir-ritonavir in patients hospitalised with COVID-19: a target trial emulation study. Lancet Infect Dis. 2024;24:1213–24. [DOI] [PubMed] [Google Scholar]
- 19.Drysdale M, Berktas M, Gibbons DC, Rolland C, Lavoie L, Lloyd EJ. Real-world effectiveness of sotrovimab for the treatment of SARS-CoV-2 infection during Omicron BA.2 and BA.5 subvariant predominance: a systematic literature review. Infection. 2024;52:1839–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell CF, Gibbons DC, Drysdale M, et al. Real-world effectiveness of sotrovimab in preventing hospitalisation and mortality in high-risk patients with COVID-19 in the United States: a cohort study from the Mayo Clinic electronic health records. PLoS ONE. 2024;19:e0304822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajme-López S, Martinez-Guerra BA, Román-Montes CM, et al. Nirmatrelvir/ritonavir and remdesivir against symptomatic treatment in high-risk COVID-19 outpatients to prevent hhospitalisationor death during the Omicron era: a propensity score-matched study. Ther Adv Infect Dis. 2024;11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyerowitz EA, Li Y. The landscape of antiviral therapy for COVID-19 in the era of widespread population immunity and omicron-lineage viruses. Clin Infect Dis. 2024;78:908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calderón-Parra J, Gutiérrez-Villanueva A, Ronda-Roca G, et al. Efficacy and safety of antiviral plus anti-spike monoclonal antibody combination therapy vs. monotherapy for high-risk immunocompromised patients with mild-to-moderate SARS-CoV2 infection during the Omicron era. A prospective cohort study. Int J Antimicrob Agents. 2024;63:107095. [DOI] [PubMed] [Google Scholar]
- 24.Rotundo S, Berardelli L, Gullì S, et al. Early initiation of combined therapy in severely immunocompromised patients with COVID-19: a retrospective cohort study. BMC Infect Dis. 2024;24:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinsella PM, Moso MA, Morrissey CO, Dendle C, Guy S, Bond K, et al. Antiviral therapies for the management of persistent coronavirus disease 2019 in immunocompromised hosts: a narrative review. Transpl Infect Dis. 2024;26:e14301. [DOI] [PubMed] [Google Scholar]
- 26.Wu TY, Chen PY, Wang JT, Liu WD, Chen YC, Chang SC. Suboptimal response to combination therapy with tixagevimab/cilgavimab and remdesivir for persistent SARS-CoV-2 infections in immunocompromised patients. J Antimicrob Chemother. 2024;79:1196–200. [DOI] [PubMed] [Google Scholar]
- 27.Visentin A, Pickavance E, San-Juan R, Grossi PA, Manuel O, Aguado JM. Current management of SARS-CoV-2 infection in solid organ transplant recipients: experience derived from an ESGICH-ESOT survey. Transpl Infect Dis. 2024;26:e14252. [DOI] [PubMed] [Google Scholar]
- 28.Royal College of Physicians. National Early Warning Score (NEWS) 2: SStandardisingthe assessment of acute-illness severity in the NHS. Updated report of a working party, RCP, London. 2017. Available at: https://www.rcp.ac.uk/media/a4ibkkbf/news2-final-report_0_0.pdf (accessed Dec 2024).
- 29.ISARIC 4 C Mortality Score. Available at: https://isaric4c.net/risk/4c/ (accessed Dec 2024).
- 30.Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalised patients with COVID-19. JAMA Intern Med. 2020;180:1081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Battaglini D, Lopes-Pacheco M, Castro-Faria-Neto HC, Pelosi P, Rocco PRM. Laboratory biomarkers for diagnosis and prognosis in COVID-19. Front Immunol. 2022;13:857573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulloque-Badaracco JR, Ivan Salas-Tello W, Al-Kassab-Córdova A, et al. Prognostic value of neutrophil-to-lymphocyte ratio in COVID-19 patients: a systematic review and meta-analysis. Int J Clin Pract. 2021;75:e14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poudel A, Poudel Y, Adhikari A, Aryal BB, Dangol D, Bajracharya T, et al. D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hhospitalisedpatients with COVID-19. PLoS ONE. 2021;16:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker KL, Nylén ES, White JC, Müller B, Snider RH. Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89:1512–25. [DOI] [PubMed] [Google Scholar]
- 35.Shen Y, Cheng C, Zheng X, et al. Elevated procalcitonin is positively associated with the severity of COVID-19: a meta-analysis based on 10 cohort studies. Medicina. 2021;57:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aziz M, Fatima R, Lee-Smith W, Assaly R. The association of low serum albumin level with severe COVID-19: a systematic review and meta-analysis. Crit Care. 2020;24:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu F, Chen R, Xu K, et al. Liver dysfunction on admission worsens clinical manifestations and outcomes of coronavirus disease 2019. Chin Med J Pulm Crit Care Med. 2023;1:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu L, Ying Y, Xie J, et al. Contributing factors affecting the length of hospital stay among febrile patients with Omicron reported in Suzhou. Clin Lab. 2024;1:70. [DOI] [PubMed] [Google Scholar]
- 39.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan H, Zhou L, Lv J, et al. Bacterial coinfections contribute to severe COVID-19 in winter. Cell Res. 2023;33:562–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gan Y, Zhang G, Sun H, Lyu X. Clinical characteristics and risk factors for bacterial co-infections in COVID-19 patients: a retrospective study. J Glob Antimicrob Resist. 2024;38:6–11. [DOI] [PubMed] [Google Scholar]
- 42.Westblade LF, Simon MS, Satlin MJ. Bacterial coinfections in Coronavirus Disease 2019. Trends Microbiol. 2021;29:930–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bordino V, Vicentini C, D’Ambrosio A, Quattrocolo F, Collaborating G, Zotti CM. Burden of healthcare-associated infections in Italy: incidence, attributable mortality and disability-adjusted life years (DALYs) from a nationwide study, 2016. J Hosp Infect. 2021;113:164–71. [DOI] [PubMed] [Google Scholar]
- 44.Sands KE, Blanchard EJ, Fraker S, Korwek K, Cuffe M. Health care-associated infections among hospitalised patients with COVID-19, March 2020-March 2022. JAMA Netw Open. 2023;6:e238059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patton MJ, Orihuela CJ, Harrod KS, et al. COVID-19 bacteremic co-infection is a major risk factor for mortality, ICU admission, and mechanical ventilation. Crit Care. 2023;27:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brosh-Nissimov T, Hussein K, Wiener-Well Y, et al. HHospitalisedpatients with severe Coronavirus Disease 2019 during the Omicron wave in Israel: benefits of a fourth vaccine dose. Clin Infect Dis. 2023;76:e234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solante R, Alvarez-Moreno C, Burhan E, et al. Expert review of global real-world data on COVID-19 vaccine booster effectiveness and safety during the omicron-dominant phase of the pandemic. Expert Rev Vaccines. 2023;22:1–16. [DOI] [PubMed] [Google Scholar]
- 48.Link-Gelles R, Levy ME, Natarajan K, et al. Estimation of COVID-19 mRNA vaccine effectiveness and COVID-19 illness and severity by vaccination status during Omicron BA.4 and BA.5 sublineage periods. JAMA Netw Open. 2023;6:e232598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeCuir J, Surie D, Zhu Y, et al. Effectiveness of original monovalent and bivalent COVID-19 vaccines against COVID-19-associated hospitalisation and severe in-hospital outcomes among adults in the United States, September 2022-August 2023. Influenza Other Respir Viruses. 2024;18:e70027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serrano-Ortiz Á, Romero-Cabrera JL, Monserrat Villatoro J, et al. Assessing COVID-19 vaccine effectiveness and risk factors for severe outcomes through machine learning techniques: a real-world data study in Andalusia, Spain. J Epidemiol Glob Health. 2024;14:1504–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


