Abstract
Background
Previous studies found that it is promising to achieve the protective effects of dietary patterns on cardiovascular health through the modulation of gut microbiota. However, conflicting findings have been reported on how dietary patterns impact gut microbiota in individuals either established or at risk of cardiovascular disease (CVD). Our systematic review aimed to explore the effect of dietary patterns on gut microbiota composition and on risk factors for CVD in these populations.
Methods
We systematically searched seven databases, including PubMed/MEDLINE, MEDLINE (Ovid), Embase (Ovid), CINHAL (EBSCO), Web of Science, CNKI (Chinese), and Wanfang (Chinese), covering literature from inception to October 2024. Studies were included if they focused on adults aged 18 years and older with CVD or at least two CVD risk factors, implemented dietary pattern interventions, and incorporated outcomes related to microbiome analysis. The risk of bias for included studies was assessed using the revised Cochrane risk of bias tool (RoB2) for randomized trials and the Risk Of Bias In Non-randomised Studies of Interventions (ROBINS-I) for non-randomized studies. Changes in the relative abundance of the gut microbiome were summarized at various taxonomic levels, including phylum, class, order, family, genus, and species. Random-effects meta-analysis was conducted to analyze the mean difference in cardiometabolic parameters pre- and post-intervention.
Results
Nineteen studies were identified, including 17 RCT and two self-controlled trails. Risk of bias across the studies was mixed but mainly identified as low and unclear. The most frequently reported increased taxa were Faecalibacterium (N = 8) with plant-rich diets, Bacteroides (N = 3) with restrictive diets, and Ruminococcaceae UCG 005 and Alistipes (N = 9) with the polyphenol-rich diets. The most frequently reported decreased taxa were Parabacteroides (N = 7) with plant-rich diets, Roseburia (N = 3) with restrictive diets, and Ruminococcus gauvreauii group (N = 6) with the polyphenol-rich diets. Plant-rich diets showed a significant decrease in total cholesterol (TC) with a mean difference of -6.77 (95% CI, -12.36 to -2.58; I2 = 84.7%), while restrictive diets showed a significant decrease in triglycerides (TG) of -22.12 (95% CI, -36.05 to -8.19; I2 = 98.4%).
Conclusions
Different dietary patterns showed distinct impacts on gut microbiota composition. Plant-rich diets promoted the proliferation of butyrate-producing bacteria, suggesting promising prospects for modulating gut microbiota and butyrate production through dietary interventions to enhance cardiovascular health. Further research is warranted to investigate the long-term effects of dietary patterns on clinical endpoints, such as CVD events or mortality.
Review registration
Registration number: CRD42024507660
Supplementary Information
The online version contains supplementary material available at 10.1186/s12937-024-01060-x.
Keywords: Cardiometabolic, Cardiovascular disease, Dietary pattern, Gut microbiota, Systematic review
Background
Cardiovascular disease (CVD) remains the leading cause of global mortality, contributing significantly to reduced quality of life and excess health system costs [1]. According to the Global Burden Disease Study, cases of total CVD, CVD-related deaths, and years lived with disability doubled from 1990 to 2019. Concurrently, disability-adjusted life years (DALYs) and years of life lost among CVD patients also increased significantly [1]. Amidst its multifaceted etiology, which includes environmental, metabolic, and behavioral risk factors, the crucial role of dietary patterns in the management of CVD has garnered increasing recognition [2]. Dietary patterns are defined as the amounts, proportions, variety, or combinations of different foods, beverages, and nutrients in the diet, and the frequency with which they are habitually consumed [3]. A healthy dietary pattern consists of nutrient-dense forms of foods and beverages across all food groups, in recommended amounts, and within calorie limits [3]. Unhealthy dietary patterns accounted for 6.58 million cardiovascular deaths (95% CI: 2.27–9.52 million) and contributed to an all-cause DALYs rate of 2,340 per 100,000 (95% CI: 836–3,380 per 100,000) in 2021 [4]. There is an urgent need for comprehensive strategies aimed at mitigating the burden of CVD through targeted dietary interventions [5, 6].
All individuals, regardless of their CVD risk status, can benefit from healthy diet, which focuses on increasing consumption of fruits, vegetables, whole grains, fat-free or low-fat dairy, lean proteins, and oils, and on decreasing consumption of foods high in sodium, saturated or trans fats, and added sugars [7]. Given the growing knowledge of the synergy between nutrients and their food sources, dietary guidelines have shifted from focusing on isolated nutrients to broader dietary patterns. Previous studies have highlighted the potential cardiovascular health benefits associated with dietary patterns such as the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), vegetarian, Nordic, and low-fat diets, while also indicating that Western and high-fat diets may elevate the risk of CVD [8–10]. It is evident that multiple dietary patterns can be beneficial for cardiovascular health, although there is significant variability in the outcomes of dietary interventions.
The existence of the Gut-Heart Axis makes this issue more complicated. Gut microbiota play an important role in the digestive process and contribute to the absorption of nutrients and metabolites, potentially modulating host immune and metabolic functions, and influencing cardiovascular health [11]. An imbalance in the gut microbiota composition has been linked to a higher risk of major cardiovascular events, such as atherosclerosis, heart failure, and stroke [12, 13]. The gut microbiota can both influence and be influenced by virtually all known cardiovascular risk factors [12]. Diet is one of the most important modulators of gut microbiota composition and function [14]. Recent studies have implicated a potential link between dietary patterns, gut microbiota, and cardiovascular health outcomes, suggesting that the gut microbiome may mediate the beneficial effects of specific dietary patterns on CVD risk [15, 16]. It is promising to achieve the protective effects of dietary patterns on cardiovascular health through the modulation of gut microbiota.
However, current findings on the impact of dietary patterns on gut microbiota present conflicting results. Santos-Marcos et al. [17] observed a decrease in the levels of Roseburia with the Mediterranean diet, while other studies reported an increase [16, 18]. Additionally, Ghosh et al. [18] noted an increase in Bacteroides following the Mediterranean diet, whereas Di et al. [19] found results contradicting this observation. How diet patterns influenced the gut microbiota to improve cardiovascular health remains unclear.
Therefore, the aim of our systematic review and meta-analysis was to explore the effects of dietary patterns on both the relative abundance of gut microbiota and key microbiota-mediated CVD risk factors in individuals with established CVD or at risk of CVD. By synthesizing existing literature, we hope to identify dietary strategies that may mitigate CVD risk by modulating gut microbiota composition and function.
Methods
The Joanna Briggs Institute (JBI) Reviewer’s Manual [20] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21] were employed to guide the methodology and reporting of this systematic review. The review was registered prior to its start in the PROSPERO international prospective register of systematic reviews (CRD42024507660).
Search strategy
A three-step comprehensive search strategy was conducted to identify both published and gray literatures in English and Chinese. The initial search was performed on PubMed/MEDLINE to identify synonyms and index terms, with the aim of formulating the final search strategy for all databases. After the final search strategy gained approved from all authors and a librarian, a comprehensive search was independently performed by two authors (JY & YW) across several databases, including PubMed/MEDLINE, MEDLINE (Ovid), Embase (Ovid), CINHAL (EBSCO), Web of Science, CNKI (Chinese), and Wanfang (Chinese). Grey literature was searched via Google Scholar and Baidu Scholar (Chinese). In PubMed/MEDLINE, we combined MeSH terms (“Diet” AND “Gastrointestinal Microbiome” AND “Cardiovascular Diseases”) with free words [(“Dietary pattern” OR “Dietary intervention” OR “Diet management”) AND (“gut microbio*” OR “gut intestinal microbio*” OR “gastrointestinal flora”) AND (“cardiovascular disease*” OR “atheroscleros*” OR “heart disease*”)]. The search time frame was set from inception to October 2024. The search strategies used for all databases are presented in Supplementary Table S1. Finally, two authors (JY & YW) conducted an extra search through manual inspection of the references in all included studies.
Inclusion and exclusion criteria
Studies were included if they: (1) focused on populations aged 18 years and older with cardiovascular diseases (CVD) or those at risk for CVD [7] (possessing at least two CVD risk factors). CVD encompassed conditions such as atherosclerosis, coronary heart disease, myocardial infarction, stroke, acute coronary syndrome, and carotid endarterectomy. The recommendation statement from the US Preventive Services Task Force suggests that individuals at increased risk of CVD should be defined as having two or more risk factors, including hypertension or elevated blood pressure, dyslipidemia, abnormal blood glucose levels, and overweight/obesity [7]. (2) implemented dietary pattern interventions in the intervention and/or control groups. (3) incorporated outcomes comprising microbiome analysis, such as 16S rRNA gene sequencing or shotgun metagenomic sequencing. (4) were randomized or nonrandomized clinical trials. (5) were published in English or Chinese. Studies involving interventions with prebiotics, probiotics, or synbiotics were excluded.
Study screening and selection
All records identified from the databases were imported into EndNote (Clarivate Analytics, PA, USA). After removing duplicates, the screening process consisted of two phases. In the primary screening, two reviewers (JY & YW) independently assessed the titles and abstracts of the records against the predefined inclusion and exclusion criteria. This initial review aimed to identify potentially relevant studies for further evaluation. In the secondary screening, the same reviewers conducted a detailed assessment of the full texts of the selected studies to confirm eligibility based on the inclusion criteria. Any discrepancies between the reviewers’ decisions during this stage were discussed until a consensus was reached. The reasons for exclusion at this stage were documented to ensure transparency in the selection process.
Risk of bias appraisal
The risk of bias of included studies was assessed using the revised Cochrane risk of bias tool (RoB2) for randomized trails and the Risk Of Bias In Non-randomised Studies—of Interventions (ROBINS-I) for non-randomized studies of interventions [22, 23]. Two reviewers (JY&YW) independently appraised the studies, and any disagreements were discussed to reach a consensus.
Data extraction
Data extraction was independently performed by two reviewers (JY&YW), including authors, year of publication, country, study design, study duration, participants characteristics, sample size, dietary intervention and control group diet, cardiometabolic parameter outcomes, methods of evaluating gut microbiota, and changes in gut microbiota composition.
Results were extracted when significant changes in the relative abundance of the gut microbiome were observed pre- and post-intervention. These results were then summarized at various taxonomic levels, including phylum, class, order, family, genus, and species. Due to the limited availability and absence of standard deviation for fold change values, a meta-analysis of gut microbiome composition was not feasible. To explore the effects of different dietary patterns, subgroup analyses were further conducted within the plant-rich diets (the plant-based, Mediterranean, and whole grain diets) and restrictive diets (low-fat and fasting diets).
Meta-analysis
Studies sharing the same dietary interventions and outcomes were included in the meta-analysis. To compare the effects of dietary interventions across studies, dietary patterns were categorized into three groups: plant-rich diets (including plant-based, vegetarian, 50% fruits and vegetables, Mediterranean, and whole grain diets), restrictive diets (including low-fat and fasting diets), and polyphenol-rich diets. Stata 17.0 (College Station, TX) was used to perform the analyses.
Mean difference (95% confidence interval [CI]) between baseline and post-intervention changes in cardiometabolic parameters were calculated for body mass index (BMI), weight, waist, systolic blood pressure (SBP), diastolic blood pressure (DBP), glucose, total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), and triglycerides (TG). We employed the DerSimonian and Laird weighted random effects model to estimate the pooled results of cardiometabolic outcomes, which is particularly suitable for our analysis due to the heterogeneity among the included studies regarding dietary interventions and participant characteristics [24]. Statistical heterogeneity among the included studies was estimated using the I2 statistic. A two-sample t-test was used to evaluate the differences between the plant-rich diets group and the restrictive diets group for the above parameters. A p value of less than 0.05 indicates statistical significance.
We conducted several sensitivity analyses to check the robustness of findings. First, we performed a leave-one-out sensitivity analysis by sequentially removing each individual trial from the analysis to explore the potential influence of an outlier. Second, we used fixed-effects models to verify the robustness of the pooled results obtained from the random-effects models. A two-sample t-test was used to evaluate the differences between the results of the two models. Since there were fewer than ten trials available for the meta-analysis, Egger’s test was not conducted for assessing the risk of publication bias.
Certainty of evidence
The certainty of evidence was rated as high, moderate, low, or very low using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach [25]. Randomized and non-randomized experimental trials were initially assessed as high-quality evidence. We then used the five GRADE criteria to potentially downgrade the initial rating: risk of bias, inconsistency, indirectness, imprecision, and publication bias.
Results
Literature search
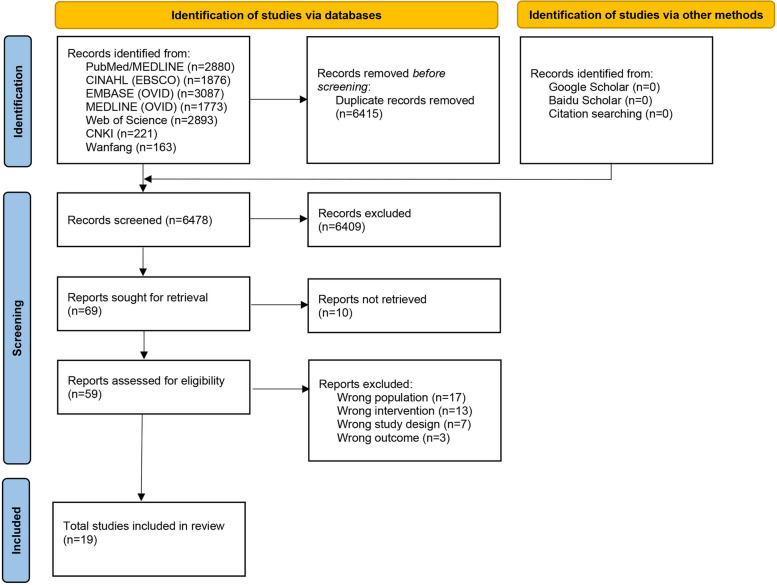
The PRISMA flowchart is shown in Fig. 1. A total of 12,893 studies were identified in the database search. After removing duplicates and initially screening titles and abstracts, 69 studies were selected. After full text review, 19 studies were included in our systematic review.
Fig. 1.
Flow diagram of the selection process
Characteristics of eligible studies
Table 1 shows the characteristics of the included studies. Among the 19 included studies, 9 were parallel RCTs [26–34], 8 were crossover RCTs [35–42], and two were self-controlled trails [43, 44]. Five studies were conducted in Spain [28–30, 32, 38], followed by the United States (n = 3) [41, 43, 44], Sweden (n = 2) [36, 37], Denmark (n = 2) [39, 42], Italy (n = 2) [34, 40], Australia (n = 1) [35], Mexico (n = 1) [26], China (n = 1) [27], Germany (n = 1) [31], and Netherlands (n = 1) [33]. The sample sizes ranged from 17 [44] to 362 [32]. The baseline age of participants ranged from a mean of 22.2 ± 3.4 years [44] to a median of 67 (63–70) years [36]. Three studies only contained male participants [29, 30, 37]. Four studies [28–30, 36] enrolled participants diagnosed with CVD diagnosed, four studies [31, 38, 39, 44] enrolled participants with metabolic syndrome, and seven studies [26, 27, 32, 34, 35, 41, 42] included participants with at least three CVD risk factors.
Table 1.
Characteristics of the included studies
| Author, year | Country | Study design | Study duration | Study population | Baseline characteristics | Intervention | Methods for measuring dietary adherence | Gut microbiota analysis method |
|---|---|---|---|---|---|---|---|---|
| Ahrens et al., 2021 | US | Self-controlled trail | 6 days | N = 73; Individuals with moderate to high ASCVD risk |
Age: 46.9 ± 12.4 years BMI: 31.1 ± 8.8 kg/m2 |
Plant-based food with minimal sugar, salt, and oil | None | 16S rRNA V3-V4 hypervariable region sequencing |
| Choo et al., 2023 | Australia | 2*2 cross-over RCT | 8 weeks per intervention, 8-week wash-out | N = 34; Adults with a risk of CVD: age between 45 and 75 years, an elevated SBP of 120 mmHg, and a diagnosis of at least two other risk factors |
Age: 61.1 ± 6.9 years BMI: 30.5 ± 3.5 kg/m2 |
Mediterranean diets supplemented with adequate dairy foods vs. low-fat control diets | Completed MedDiet adherence tool and low-fat diet adherence tool each fortnight; 3-day weighed food record | 16S rRNA V4 hypervariable region sequencing |
| Clark et al., 2019 | US | Self-controlled trail | 9 weeks | N = 17; Individuals diagnosed with MetS who have at least 3 risk factors |
Age: 22.2 ± 3.4 years BMI: 37.9 ± 5.0 kg/m2 |
Diet comprising 50% fruit and vegetable intake | Participants provided daily food and activity logs, food receipts, and food pictures weekly | 16S rRNA V3-V4 hypervariable region sequencing |
| Djekic et al., 2020 | Sweden | Crossover RCT | 4 weeks per intervention, 4-week wash-out | N = 31; Adults age > 18 years, with stable ischemic heart disease, who underwent percutaneous coronary intervention > 1 month before the study initiation, and received optimal medical therapy |
Age: 67.0 (63.0–70.0) years BMI: 27.5 ± 2.9 kg/m2 |
Lacto-ovo-vegetarian diet vs. meat diet | 3-day weighted food record | 16S rRNA V4 hypervariable region sequencing |
| Eriksen et al., 2020 | Sweden | Crossover RCT | 8 weeks per intervention, 8-week wash-out | N = 49; Men aged 49–74 years, who had at least 2 MetS risk factors |
Age: 65.0 ± 8.0 years BMI: 30.9 ± 3.3 kg/m2 |
Whole grain rye diet with 4 weeks secoisolariciresinol diglucoside lignan capsules vs. whole grain wheat diet | Weighed 3-day dietary records and personal checklists | 16S rRNA V3-V4 hypervariable region sequencing |
| Galie et al., 2021 | Spain | Crossover RCT | 8 weeks per intervention, 1 month wash-out | N = 50; Idividual aged 25–60 years with a BMI between 25 and 35 kg/m2 and diagnosed with MetS | Age: 51.4 (49.2–53.6) years | Mediterranean diet vs. a daily intake of 50 g of mixed nuts | 17-item MedDiet score, counting the empty nuts-packaging, 3-day dietary records | 16S rRNA V4 hypervariable region sequencing |
| Guevara-Cruz et al., 2019 | Mexico | Parallel RCT | 75 days | N = 113; Adults aged 20–60 years, with a BMI 25–50 kg/m2, and meeting 3 positive criteria for MetS |
Age: 40.8 ± 0.4 years; BMI: 33.8 ± 0.2 kg/m2 |
Reduced-energy low-saturated-fat diet with functional foods | 24-h diet recall, 3-day food records, and measuring the number of empty packages returned | 16S rRNA V3-V4 hypervariable region sequencing |
| Guo et al., 2021 | China | Parallel RCT | 8 weeks | N = 46; Adults aged 30–50 years, with central obesity and 2 other risk factors |
Age: 40.2 ± 5.7 years; BMI: 28.0 (25.8–32.9) kg/m2 |
Intermittent fasting (75% of energy restriction for 2 nonconsecutive days a week) | 3-day dietary records | 16S rRNA |
| Hald et al., 2016 | Denmark | Crossover RCT | 4 weeks per intervention, 4-week wash-out | N = 22; Adults aged 39–75 years with MetS |
Age: 60.0 (48.0–67.0) years; BMI: 30.6 (29.3–35.2) kg/m2 |
Healthy-carbohydrate diet with high concentration of diet fibre vs. low-fibre Western-style diet | 3-day dietary records | 16S rRNA V4 hypervariable region sequencing |
| Haro et al., 2016 | Spain | Parallel RCT | 2 years | N = 239; Patients diagnosed with coronary heart disease with or without MetS | Age: 61.4 ± 0.9 years in non-MetS group, 60.2 ± 0.7 years in MetS group | Mediterranean diet vs. low-fat high-carbohydrate diet (28% fat [12% monounsaturated, 8% polyunsaturated and 8% saturated]) | 14-item/9-point questionnaire | 16S rRNA |
| Haro et al., 2016 | Spain | Parallel RCT | 1 year | N = 20; Men with obesity and coronary heart disease |
Age: 63.3 ± 2.0 years BMI: 32.2 ± 0.5 kg/m2 |
Mediterranean diet vs. low-fat high-complex carbohydrate diet (28% fat [12% monounsaturated, 8% polyunsaturated and 8% saturated]) | None | 16S rRNA |
| Haro et al., 2017 | Spain | Parallel RCT | 2 years | N = 106; Male patients diagnosed with coronary heart disease; 33 obese people with MetS, 32 obese people without MetS, and 41 non obese people without MetS |
Age: 59.0 ± 1.8, 63.7 ± 1.7, 61.7 ± 1.4 years; BMI: 32.4 ± 0.8, 32.9 ± 0.6, 27.1 ± 0.3 kg/m2 |
Mediterranean diet vs. low-fat diet (< 30% total fat [< 10% saturated fat, 12–14 MUFA fat and 6–8% PUFA fat]) | 14-item/9-point questionnaire | 16S rRNA V4 hypervariable region sequencing |
| Maifeld et al., 2021 | Germany | Parallel RCT | 3 months | N = 35; Patients with MetS |
Age: 58.0 ± 8.0, and 62.0 ± 8.0 years; BMI: 34.0 ± 4.9, and 33.0 ± 4.7 kg/m2 |
7 days Fasting (calorie-restricted vegan diet) followed modified DASH diet vs. only modified DASH diet | None | 16S rRNA gene V4 region amplification, and shotgun metagenomic sequencing |
| Muralidharan et al., 2021 | Spain | Parallel RCT | 1 year | N = 362; Adults aged 55–75 years, who are overweight/obese and have at least 3 components of MetS |
Age: 64.3 ± 5.1, 65.1 ± 4.9 years BMI: 33.4 (30.8–36.0), 32.9 (30.5–35.6) kg/m2 |
Energy-reduced Mediterranean diet (accompanied with physical activity advice) vs. unrestricted caloric Mediterranean diet (with no advice on increase physical activity) | Mediterranean diet adherence score | 16S rRNA V4 hypervariable region sequencing |
| Pagliai et al., 2020 | Italy | Crossover RCT | 3 months per intervention, 2-week wash-out period | N = 23; Adults aged 18–75 years with a low to medium cardiovascular risk profile (1–5% according to the guidelines of the European Society of Cardiology) | Age: 58.6 ± 9.8 years | Mediterranean diet vs. Vegetarian diet | 24-h diet recall, 9-item adherence score | 16S rRNA V3-V4 hypervariable region sequencing |
| Petersen et al., 2022 | US | Crossover RCT | 4 weeks per intervention, 2-week wash-out | N = 56; Adults aged 30–75 years, with a BMI 25–35 kg/m2, abdominal obesity, and at least 1 other risk factor for CVD |
Age: 45.0 ± 11.0 years BMI: 29.8 ± 2.9 kg/m2 |
The American diet, supplemented with herbs and spices, was divided into three groups: low/moderate/high dose groups | Participants self-report | 16S rRNA V4 hypervariable region sequencing |
| Roager et al., 2019 | Denmark | Crossover RCT | 8 weeks per intervention, 6-week wash-out period | N = 60; Adults aged 20–65 years who are weight stable, with a BMI of 25–35 kg/m2 and/or increased waist circumference, and at least one other CVD risk factor |
Age: 48.6 ± 11.1 years; BMI: 28.9 ± 3.6 kg/m2 |
Whole grain diet vs. refined grain diet | Diary; measured fasting concentrations of plasma alkylresorcinols | 16S rRNA gene sequencing and shotgun metagenomic sequencing |
| Van Trijp et al., 2021 | Dutch | Parallel RCT | 12 weeks | N = 37; Adults aged 45–70 years, with a BMI 25–35 kg/m2 and an elevated plasma total cholesterol |
Age: 60 ± 5.4, and 60 ± 6.0 years; BMI: 28 ± 2.0, and 27 ± 2.2 kg/m2 |
Whole grain diet vs. refined grain diet | None | 16S rRNA V4 hypervariable region sequencing |
| Vetrani et al., 2020 | Italy | Parallel RCT | 8 weeks | N = 78; Adults aged 35–70 years, with a BMI 27–35 kg/m2, increased waist circumference, and at least one or more components of MetS |
Age: 54.0 ± 9.0. 56.0 ± 8.0, 53.0 ± 9.0, 55.0 ± 9.0 years; BMI: 33.0 ± 3.0, 32.0 ± 4.0, 32.0 ± 3.0, 30.0 ± 3.0 kg/m2 |
Four diet patterns: (a) low LCn3&PP, diet low in LCn3 (1.5 g/day) and PP (365 mg/day); (b) high LCn3, diet high in LCn3 (4 g/day) and low in PP (363 mg/day); (c) high PP, diet high in PP (2903 mg/day) and low in LCn3 (1.4 g/day); and (d) high LCn3&PP, diet high in PP (2861 mg/day) and LCn3 (4 g/day) | 7-day food records | 16S rRNA |
Abbreviation: ASCVD atherosclerotic cardiovascular disease, BMI body mass index, CVD cardiovascular disease, LCn3 long-chain n-3 polyunsaturated fatty acids, MedDiet the Mediterranean diet, MetS metabolic syndrome, PP polyphenols, RCT randomized controlled trial
Seven studies described the Mediterranean diets [28–30, 32, 35, 38, 40], followed by the low-fat diets (n = 5) [26, 28–30, 35], the plant-based diets (n = 4) [36, 40, 43, 44], the whole grain diets (n = 4) [33, 37, 39, 42], the fasting diets (n = 2) [27, 31], and the polyphenol-rich diets (n = 2) [34, 41]. Among the studies that conducted the Mediterranean diet intervention, four studies [28–30, 35] used the low-fat diet as the control group, and one [40] used the vegetarian diet as control. Among the studies conducted the whole grain diet intervention, three [33, 39, 42] of them used a refined grain diet as control, and one compared whole grain rye diet and whole grain wheat diet [37]. The intervention duration ranged from 6 days [43] to 2 years [28, 29], with 15 studies lasting no more than 3 months [26, 27, 31–44].
Eleven studies reported the outcome of cardiometabolic parameters, with the most reported measures being TC, TG, HDL-C, and LDL-C (n = 10) [26, 27, 30, 32, 36–38, 42–44] and weight (n = 9) [26, 27, 32, 36, 38, 39, 42–44]. All of the studies used 16S rRNA sequencing method for gut microbiome analysis, and 2 studies further used shotgun metagenomic sequencing [31, 42]. A variety of methods were used to calculate the α-diversity of gut microbiota, including Faith’s phylogenetic diversity (n = 8) [29, 30, 33, 35, 36, 38, 39, 41], Shannon index (n = 8) [26, 27, 31, 32, 38, 40, 42, 43], Chao 1 (n = 7) [26, 29–32, 39, 43], observed operational taxonomic units (n = 6) [26, 27, 29, 38, 39, 41], Simpson index (n = 5) [27, 31, 38, 40, 43], the Pielou’s evenness (n = 2) [35, 41], and the ACE estimator (n = 1) [43]. The β-diversity was calculated using the weighted UniFrac distance (n = 8) [26, 27, 30, 32, 33, 39–41], the unweighted UniFrac distance (n = 5) [26, 27, 29, 30, 32], and Bray–Curtis distance (n = 4) [31, 32, 38, 40].
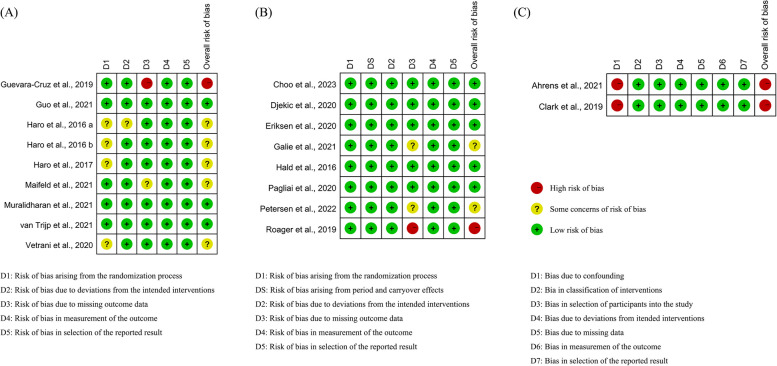
Risk of bias assessment
Figure 2 shows the results of the study’s risk of bias assessment. There were four studies [28–30, 34] demonstrated potential biases related to randomization, while three studies [31, 38, 41] exhibited concerns due to missing outcome data. Both non-RCTs inadequately controlled for significant confounding variables [43, 44].
Fig. 2.
Risk of bias of included studies. A Results of parallel randomized controlled trials; B Results of cross over randomized controlled trials; C Results of non-randomized controlled trials
Cardiometabolic parameters
Figure 3 shows the results of meta-analysis on clinical cardiometabolic outcomes. Detailed information can be found in Table S2, Table S3 and Figure S1. Ten studies, including 14 dietary intervention groups that evaluated clinical cardiometabolic outcomes, were included in our meta-analysis. Among these 14 groups, eight were plant-rich diets interventions (three plant-based diets [36, 43, 44], three Mediterranean diets [30, 32, 38], and two whole grain diets [39, 42]), while six were restrictive diet interventions (five low-fat diets [26, 30] and one fasting diets [27]). Results showed that plant-rich diets significantly reduce TC (mean difference, −6.77; 95% CI, −12.36 to −2.58; I2, 84.7%), LDL-C (−4.37, −7.59 to −1.16, 65.1%), and weight (−0.45, −0.77 to −0.13, 0.0%). Restrictive diets significantly reduce TG (−22.12, −36.05 to −8.19, 98.4%), TC (−8.74, −12.72 to −4.76, 94.1%), blood glucose (−7.13, −12.01 to −2.25, 99.4%), waist circumference (−4.25, −5.52 to −2.98, 95.7%), weight (−4.02, −5.26 to −2.77, 94.7%), and BMI (−1.59, −2.18 to −0.99, 97.7%). The restrictive diets group showed significantly greater reductions in BMI (t = 3.77, p = 0.0002), weight (t = 5.22, p < 0.0001), waist circumference (t = 4.55, p < 0.0001), and blood glucose (t = 2.25, p = 0.0248) compared to the plant-rich diets group.
Fig. 3.
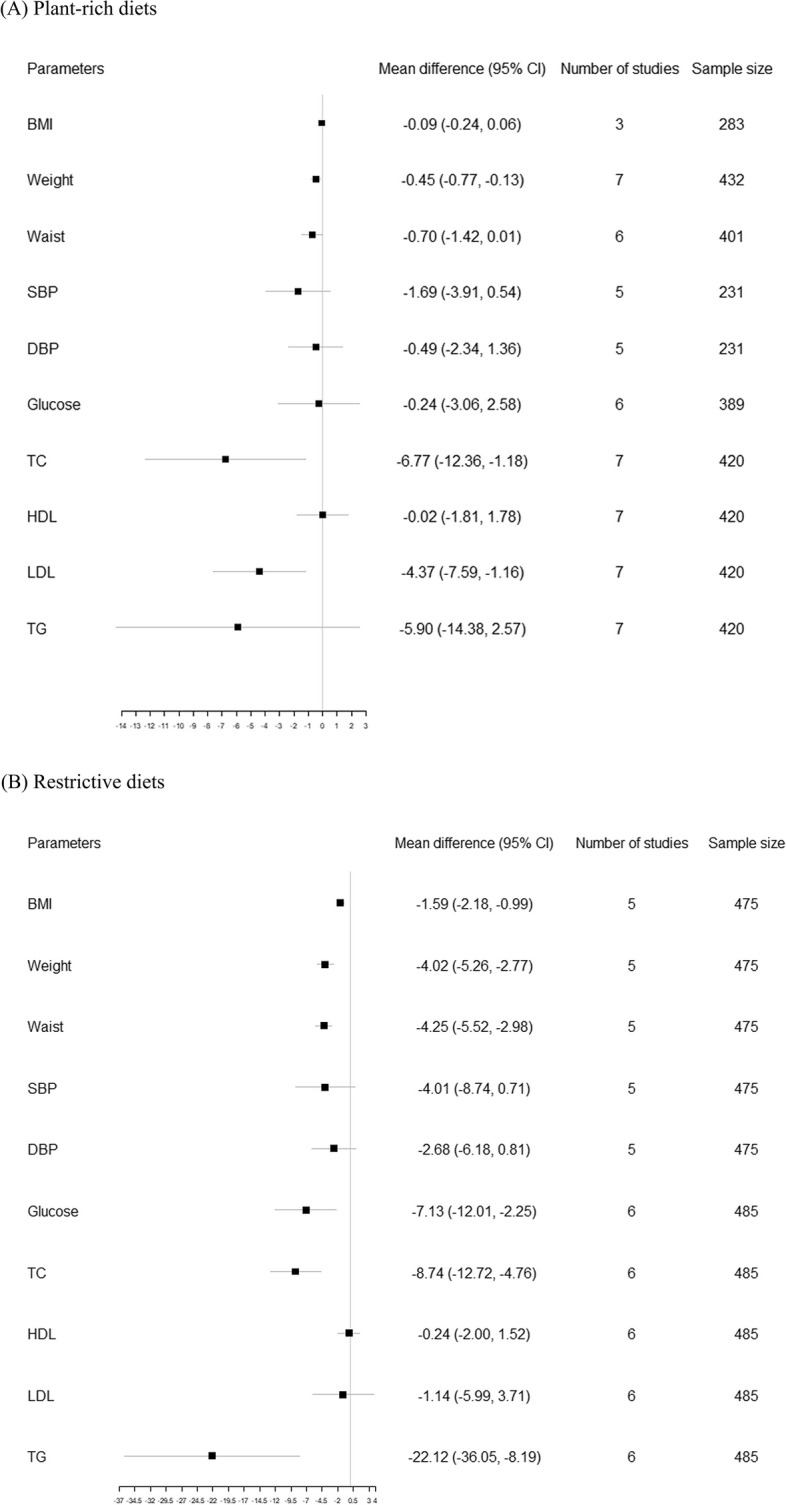
Forest plots of effects of dietary patterns on clinical parameters. A Pooled effects of plant-rich diets on clinical parameters; B Pooled effects of restrictive diets on clinical parameters
In the leave-one-out analysis, we found that no single trial significantly influenced the estimations (Figure S2). In addition, pooled results showed no significant differences between random- or fixed- effects models (Table S4).
Certainty of evidence
Table 2 shows the certainty of evidence regarding plant-rich diets and restrictive diets on cardiometabolic parameters. Reasons for the downgrade are listed in Table S5. Twelve pieces of evidence were downgraded by one level due to a high risk of bias. Additionally, ten pieces of evidence were downgraded by one level due to inconsistency, indicated by an I2 exceeding 90%. Furthermore, eight pieces of evidence were downgraded by one level due to imprecision, indicated by a sample size did not meet the optimal information size criterion or less than 400.
Table 2.
Certainty of evidence table
| Certainty assessment | Summary of findings | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | Risk of biasa | Indirectness | Inconsistency | Imprecisionb | Publication bias | GRADE | Relative effect (95%CI) | Number of participants (studies) |
| Plant-rich diets | ||||||||
| BMI | 0 | 0 | 0 | -1 | 0 | ⊕⊕⊕○ Moderate certainty evidence | -0.09 (-0.24, 0.06) | 283 (3) |
| Weight | 0 | 0 | 0 | -1 | 0 | ⊕⊕⊕○ Moderate certainty evidence | -0.45 (-0.77, -0.13) | 432 (7) |
| Waist | 0 | 0 | 0 | -1 | 0 | ⊕⊕⊕○ Moderate certainty evidence | -0.70 (-1.42, 0.01) | 401 (6) |
| SBP | -1 | 0 | 0 | -1 | 0 | ⊕⊕○○ Low certainty evidence | -1.69 (-3.91, 0.54) | 231 (5) |
| DBP | -1 | 0 | 0 | -1 | 0 | ⊕⊕○○ Low certainty evidence | -0.49 (-2.34, 1.36) | 231 (5) |
| Glucose | 0 | 0 | 0 | -1 | 0 | ⊕⊕⊕○ Moderate certainty evidence | -0.24 (-3.06, 2.58) | 389 (6) |
| TC | 0 | 0 | 0 | 0 | 0 | ⊕⊕⊕⊕ High certainty evidence | -6.77 (-12.36, -1.18) | 420 (7) |
| HDL-C | 0 | 0 | 0 | -1 | 0 | ⊕⊕⊕○ Moderate certainty evidence | -0.02 (-1.81, 1.78) | 420 (7) |
| LDL-C | 0 | 0 | 0 | 0 | 0 | ⊕⊕⊕⊕ High certainty evidence | -4.37 (-7.59, -1.16) | 420 (7) |
| TG | 0 | 0 | 0 | -1 | 0 | ⊕⊕⊕○ Moderate certainty evidence | -5.90 (-14.38, 2.57) | 420 (7) |
| Restrictive diets | ||||||||
| BMI | -1 | 0 | -1 | 0 | 0 | ⊕⊕○○ Low certainty evidence | -1.59 (-2.18, -0.99) | 475 (5) |
| Weight | -1 | 0 | -1 | 0 | 0 | ⊕⊕○○ Low certainty evidence | -4.02 (-5.26, -2.77) | 475 (5) |
| Waist | -1 | 0 | -1 | 0 | 0 | ⊕⊕○○ Low certainty evidence | -4.25 (-5.52, -2.98) | 475 (5) |
| SBP | -1 | 0 | -1 | 0 | 0 | ⊕⊕○○ Low certainty evidence | -4.01 (-8.74, 0.71) | 475 (5) |
| DBP | -1 | 0 | -1 | 0 | 0 | ⊕⊕○○ Low certainty evidence | -2.68 (-6.18, 0.81) | 475 (5) |
| Glucose | -1 | 0 | -1 | 0 | 0 | ⊕⊕○○ Low certainty evidence | -7.13 (-12.01, -2.25) | 485 (6) |
| TC | -1 | 0 | -1 | 0 | 0 | ⊕⊕○○ Low certainty evidence | -8.74 (-12.72, -4.76) | 485 (6) |
| HDL-C | -1 | 0 | -1 | 0 | 0 | ⊕⊕○○ Low certainty evidence | -0.24 (-2.00, 1.52) | 485 (6) |
| LDL-C | -1 | 0 | -1 | 0 | 0 | ⊕⊕○○ Low certainty evidence | -1.14 (-5.99, 3.71) | 485 (6) |
| TG | -1 | 0 | -1 | 0 | 0 | ⊕⊕○○ Low certainty evidence | -22.12 (-36.05, -8.19) | 485 (6) |
Abbreviation: BMI body mass index, CVD cardiovascular disease, DBP diastolic blood pressure, HDL-C high-density lipoprotein, LDL-C low density lipoprotein, SBP systolic blood pressure, TC total cholesterol, TG triglyceride
aDowngrade by one or two level if the included studies were at high risk of bias
bDowngrade by one level if the sample size did not meet the optimal information size criterion or if it is less than 400
Microbiome results
Changes in the relative abundance of gut microbiota were extracted from 17 studies [27–37, 39–44]. Table S6 and Figure S3-10 show detailed information on significant changes in the gut microbiome following dietary interventions.
Increase in relative abundance following diet interventions
At the phylum level, changes in Firmicutes phylum were the most frequently reported across all three dietary patterns (N = 66 for plant-rich diets [28–30, 32, 35–37, 40, 42, 43], 18 for restrictive diets [27, 29–31], and 69 polyphenol-rich diets [34, 41]). Compared to the other two dietary patterns, the plant-rich diets increased the relative abundance of Euryarchaeota (N = 3) and Verrucomicrobia (N = 1), while the restrictive diets increased Lactococcus (N = 1) and Spirochaetes (N = 1), and the polyphenol-rich diets increased Tenericutes (N = 5).
At the order level, all three dietary patterns increased the relative abundance of Bacteroidales, Clostridiales, Coriobacteriales, Eubacteriales, Lactobacillales, and Oscillospirales. Compared to the other two dietary patterns, the plant-rich diets increased the relative abundance of Burkholderiales (N = 1), Caulobacterales (N = 1), Eggerthellales (N = 3), Methanobacteriales (N = 2), Monoglobales (N = 2), Veillonellales (N = 2) and Verrucomicrobiales (N = 1), while the restrictive diets increased Actinomycetales (N = 1), Erysipelotrichales (N = 1), Flavobacteriales (N = 1), Pasteurellales (N = 1) and Pseudomonadales (N = 3), and the polyphenol-rich diets increased Desulfovibrionales (N = 4), Mollicutes (N = 3), and Rhodospirillales (N = 1). The other two dietary patterns increased the abundance level of Bifidobacteriales (N = 3 and 1), except the polyphenol-rich diets.
At the genus level, the most frequently reported increased taxa were Faecalibacterium (N = 8) [28, 29, 42, 43] and Roseburia (N = 6) [29, 30, 32, 37, 43] following the plant-rich diets, Bacteroides (N = 3) [29, 31] and Prevotella (N = 3) [28–30] following the restrictive diets, and Ruminococcaceae UCG 005 (N = 9) [41], Alistipes (N = 9) [41], and Ruminococcus (N = 8) [41] following the polyphenol-rich diets.
Decrease in relative abundance following diet interventions
At the phylum level, all three dietary patterns resulted in a decreased relative abundance of taxa within Actinomycetota, Bacteroidota, Firmicutes, and Proteobacteria. Compared to the other two dietary patterns, the plant-rich diets reduced the relative abundance of Cyanobacteria (N = 1) and Lentisphaerota (N = 1), while the restrictive diets reduced Acidobacteria (N = 2) and Chloroflexi (N = 4).
At the order level, the most frequently reported decreased taxa were observed in Bacteroidales (N = 29) [30, 32, 36, 39, 40, 42, 43] following the plant-rich diets, and Clostridiales following restrictive diets (N = 13) [27, 29–31] and polyphenol-rich diets (N = 18) [34, 41]. Compared to the other two dietary patterns, the plant-rich diets reduced the relative abundance of Acidaminococcales (N = 3), Burkholderiales (N = 3), Desulfovibrionales (N = 3), Peptostreptococcales (N = 2) and Victivallales (N = 1), while the restrictive diets reduced Acidothermales (N = 2), Actinomycetales (N = 2), Hyphomicrobiales (N = 1), Ktedonobacterales (N = 2), Pasteurellales (N = 1), and Solibacterales (N = 1), and the polyphenol-rich diets reduced Selenomonadales (N = 2).
At the genus level, the most frequently reported decreased taxa were Parabacteroides (N = 7) [32, 36, 39, 40, 43], Ruminococcus (N = 4) [32, 33, 39, 42], Lachnospira (N = 4) [37, 39], and Bacteroides (N = 4) [39, 43] following the plant-rich diets, Roseburia (N = 3) [30, 31], Alistipes (N = 2) [27, 31], Clostridium (N = 2) [29, 31], and Eubacterium (N = 2) [31] following the restrictive diets, and Ruminococcus gauvreauii group (N = 6) [41], Ruminiclostridium (N = 5) [41], and Ruminococcaceae UCG 013 (N = 5) [41] following the polyphenol-rich diets.
Subgroup analysis
Within the plant-rich diets, the most frequently reported increased taxa were Blautia (N = 3) [43] and Anaerostipes (N = 3) [40, 43] following the plant-based diets, Roseburia (N = 3) [29, 30, 32], Parabacteroides (N = 3) [28–30], and Faecalibacterium (N = 3) [28, 29] following the Mediterranean diets, and Faecalibacterium (N = 3) [42] and Coprococcus (N = 2) [37, 42] following the whole grain diets. The most frequently reported decreased taxa were Parabacteroides (N = 4) [36, 43] and Bacteroides (N = 3) [43] following the plant-based diets, Parabacteroides (N = 2) [32, 40] for the Mediterranean diets, and Lachnospira (N = 4) [37, 39] and Ruminococcus (N = 3) [39, 42] following the whole grain diets.
Within the restrictive diets, the most frequently reported increased taxa were Prevotella (N = 3) [28–30] following the low-fat diets, and Roseburia (N = 2) [27, 31], Clostridium (N = 2) [31], and Bacteroides (N = 2) [31] following the fasting diets. The most frequently reported decreased taxa were Streptococcus (N = 1) [29], Roseburia (N = 1) [30], and Clostridium (N = 1) [29] following the low-fat diets, and Roseburia (N = 2) [31], Eubacterium (N = 2) [31], and Alistipes (N = 2) [27, 31] following the fasting diets.
Discussion
This is the first systematic review to provide a comprehensive understanding of the impact of dietary patterns on gut microbiota and cardiometabolic indicators in individuals with established CVD or at risk of CVD. Our findings highlighted the distinct effects of plant-rich, restrictive, and polyphenol-rich dietary patterns on the composition of gut microbiota. A plant-rich diet was shown to increase the relative abundance of butyrate-producing bacteria, including Faecalibacterium prausnitzii and Roseburia, which are known to play protective roles in various forms of CVD. Additionally, both restrictive diets and plant-rich diets significantly reduced cardiometabolic risk factors, with restrictive diets showing greater efficacy. These results underscore the potential of dietary interventions to modulate gut microbiota and improve both primary and secondary prevention of CVD.
Our results indicated that plant-rich diets may lead to an increase in the relative abundance of Faecalibacterium. This aligns with findings from previous studies [45, 46]. Furthermore, our subgroup analysis revealed that this increase mainly occurred in individuals following whole grain and Mediterranean diets. This may be because these two dietary patterns are rich sources of resistant starch (RS), which is found abundantly in whole grains, chickpeas, and tuberous vegetables. RS, a type of fiber resistant to digestion in the small intestine, has been previously found to be associated with the proliferation of Faecalibacterium [47]. It suggests that plant-rich diets could be considered as an intervention strategy for individuals who require modulation of Faecalibacterium abundance. However, as highlighted by a recent systematic review, the effects of the Mediterranean diet and other plant-rich diets on gut microbiota composition remain inconclusive due to substantial heterogeneity in study design and findings [48]. This suggests that while plant-rich diets hold promise as an intervention strategy for modulating Faecalibacterium abundance, the effects may vary depending on the specific context and individual variability.
Among restrictive diets, the fasting diet was shown to increase the relative abundance of Bacteroides among individuals with metabolic syndrome, aligning with findings observed in healthy adults with normal or obese BMI [49]. Additionally, we identified a study reporting an increase in Bacteroides abundance following a low-fat diet intervention [29]. However, contrasting this observation, prior evidence suggested that animal-based or typical Western diets, characterized by their high protein and fat content, were associated with elevated Bacteroides abundance [50, 51]. This discrepancy may be attributed to individual variations and racial/ethnic diversity. Moreresearch is warranted to elucidate the impact of low-fat diets on Bacteroides abundance and the underlying mechanisms.
A plant-rich diet was found to exert beneficial effects on the proliferation of butyrate-producing bacteria, notably Faecalibacterium prausnitzii and Roseburia. This is partially supported by the findings of a previous systematic review, which suggested that while the Mediterranean diet may influence bacterial abundance and fecal butyrate concentrations [48]. Faecalibacterium prausnitzii and Roseburia, which belong to the Firmicutes phylum, were the most frequently reported to increase significantly following plant-rich dietary interventions. These bacteria may degrade the cellulose and hemicellulose components of plant material, which are subsequently fermented into short-chain fatty acids (SCFAs), including butyrate [52]. Faecalibacterium prausnitzii, in particular, is recognized as one of the most abundant producers of butyrate, a crucial SCFA [53]. There is substantial evidence suggesting that SCFAs, particularly butyrate, play important roles in various CVD [54, 55]. Reduced production of butyrate and lower abundance of Faecalibacterium have been observed in hypertensive patients [56]. Furthermore, butyrate could inhibit the development of atherosclerosis by enhancing plaque stability and reducing the adhesion and migration of pro-inflammatory macrophages [57]. The Roseburia-fiber-butyrate axis could reduce atherosclerotic plaque sizes without impacting cholesterol and triglyceride levels. Additionally, it interacts with dietary plant polysaccharides to mitigate systemic inflammation and improve atherosclerosis [58]. Butyrate also plays a vital role in reducing circulating cholesterol levels by stimulating the secretion of lipoproteins containing apoA-IV, thereby facilitating reverse cholesterol transport [59]. Additionaly, Roseburia can activate fatty acid oxidation and de novo synthesis, while inhibiting lipolysis, leading to reduced circulating lipid plasma levels and body weight [60]. These findings highlight the potential cardioprotective effects of a plant-rich diet mediated by the modulation of gut microbiota and SCFA production. It is promising to manipulate SCFA production through dietary interventions to decrease the potential cardiovascular risk for both healthy individuals and patients with existing cardiovascular conditions. Further research is warranted to elucidate the underlying mechanisms and assess the clinical implications of plant-rich diets in the prevention and management of CVD.
Our findings revealed that both restrictive diets and plant-rich diets significantly reduced TC, LDL-C, and body weight. Notably, restrictive diets were more effective in lowering BMI, weight, waist circumference, and blood glucose levels among individuals with established CVD or at risk of CVD. This aligns with a previous umbrella review, which demonstrated the association of intermittent fasting with successful weight loss and metabolic benefits in adults with obesity [61]. Additionally, low-fat diets appear to be more effective for weight loss and reducing both systolic and diastolic blood pressure compared to standard diets, and they outperform low-carbohydrate diets in lowering LDL-C levels [62]. The benefits of low-fat diets may be due to the reduction of high-energy-dense foods, such as fats, which helps lower total energy intake, a key factor for weight loss [63]. Studies suggest that low-energy–density diets are effective in reducing weight, lowering inflammation, and improving cardiovascular risk factors [64]. However, simply restricting high-energy-dense foods without increasing low-energy-dense alternatives may result in greater hunger and cravings, making it harder to sustain the diet in the long term [63]. While low-fat diets have their advantages, they can also lower HDL-C, which is associated with increased coronary heart disease risk [65]. Additionally, the reduction in fat intake often leads to an increase in carbohydrate consumption, which can trigger carbohydrate-induced hypertriglyceridemia [66]. This potential downside of a simple restrictive diet diets should be considered when evaluating their effectiveness for long-term cardiovascular prevention. Therefore, we cannot unequivocally recommend restrictive diets as the optimal strategy for CVD prevention without considering these limitations.
Our analysis indicated that plant-rich diets have relatively lower efficacy in reducing cardiometabolic risk factors compared to low-fat diets. This may be attributed to the absence of energy intake restriction in the included studies. Previous studies have found that diets rich in fruits and vegetables, without a compensatory reduction in total energy intake, may not lead to significant weight loss [67]. Reductions in waist circumference and visceral fat were primarily observed when Mediterranean diets were energy-restricted, with even then, the effect sizes were only marginal [68]. Similarly, a long-term intervention with an unrestricted-calorie, high-vegetable-fat Mediterranean diet resulted in slight reductions in bodyweight and an increase in waist circumference [69]. However, it is important to emphasize that healthy plant foods, such as extra virgin olive oil and nuts, may still be associated with a substantially lower risk of CVD [70]. The high-fiber foods in plant-rich diets may decrease the level of inflammatory biomarkers [71], which are associated with improved endothelial function and a lower risk of atherosclerosis, ultimately benefiting cardiovascular health. The U.S. Dietary Guidelines Advisory Committee advocates the Mediterranean and vegetarian diets as optimal dietary patterns for preventing common chronic non-communicable diseases [72]. For individuals who do not seek weight loss, the Mediterranean diets may still offer cardiovascular health benefits. In contrast, restrictive diets, such as low-fat diets and fasting, may inherently promote greater reductions in cardiometabolic risk factors by reducing overall energy intake. While the studies included in this analysis did not explicitly report energy intake, it is plausible that low-fat diets, due to their reduction in the consumption of calorie-dense fats, and fasting diets, by limiting the frequency of food intake, lead to lower caloric consumption, thereby enhancing their effectiveness in improving metabolic health. Therefore, the findings from our study suggest that both plant-rich diets and restrictive diets hold promise as viable dietary patterns for losing weight and improving cardiometabolic health. Combining plant-rich diets with calorie restriction may offer synergistic benefits, potentially amplifying their effectiveness in promoting weight loss and improving metabolic parameters. Future research, particularly RCTs, should investigate the long-term effects of these dietary patterns on clinical endpoints such as CVD events or mortality.
Limitations
First, our meta-analysis was restricted to clinical parameters due to the limited availability of fold change values for gut microbiota and the absence of their standard deviations. Consequently, we could only describe gut microbiota alterations focusing on the occurrence of changes and the frequency of significant alterations, rather than quantitatively assessing the absolute extent of microbiota changes. Second, the heterogeneity among the included studies in terms of interventions and control groups makes it challenging to provide definitive recommendations on which dietary pattern-induced gut microbiota changes are most advantageous for cardiovascular health. Third, our inclusion criteria limited studies to those published in English and Chinese, thus caution is advised when generalizing the conclusions.
Conclusion
Our systematic review highlighted the different impacts of plant-rich diets, restrictive diets, and polyphenol-rich diets on gut microbiota in individuals with established CVD or at risk of CVD. The plant-rich diets were shown to promote the proliferation of butyrate-producing bacteria Faecalibacterium prausnitzii and Roseburia. Butyrate plays an important role in inhibiting the development of various CVD and in reducing circulating cholesterol levels. It is promising to increase the production of butyrate through dietary interventions to reduce potential cardiovascular risks. Policymakers should prioritize and promote plant-rich diets through public health campaigns and nutritional guidelines. Furthermore, the restrictive diets showed more effective in improving cardiometabolic factors compared to the plant-rich diets. However, when using low-fat diets for CVD prevention, it is important to consider their potential limitations and long-term effects. Further research is warranted to elucidate the underlying mechanisms and investigate the long-term effects of dietary patterns on clinical endpoints such as CVD events or mortality.
Supplementary Information
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- CVD
Cardiovascular disease
- DALYs
Disability-adjusted life years
- DBP
Diastolic blood pressure
- GRADE
The Grading of Recommendations, Assessment, Development, and Evaluations
- HDL-C
High-density lipoprotein-cholesterol
- LDL-C
Low-density lipoprotein-cholesterol
- SBP
Systolic blood pressure
- TC
Total cholesterol
- TG
Triglycerides
Authors’ contributions
JY, ZZ, and HL designed research; JY and YW conducted research and analyzed data; JY and YW wrote paper; ZZ and HL reviewed and edited the paper. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 72104051], China Medical Board Open Competition Program [grant numbers #20–372], and the Shenzhen Third People’s Hospital project (No. 21250G1001; No. 22240G1005; No. XKJS-CRGRK-008).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zheng Zhu, Email: zhengzhu@fudan.edu.cn.
Hongzhou Lu, Email: luhongzhou@fudan.edu.cn.
References
- 1.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J Am Coll Cardiol. 2020;76(25):2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. December 2020. Available at https://www.dietaryguidelines.gov/.
- 4.Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The Global Burden of Cardiovascular Diseases and Risk. J Am Coll Cardiol. 2022;80(25):2361–71. [DOI] [PubMed] [Google Scholar]
- 5.Kaminsky LA, German C, Imboden M, Ozemek C, Peterman JE, Brubaker PH. The importance of healthy lifestyle behaviors in the prevention of cardiovascular disease. Prog Cardiovasc Dis. 2022;70:8–15. [DOI] [PubMed] [Google Scholar]
- 6.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. Behavioral Counseling Interventions to Promote a Healthy Diet and Physical Activity for Cardiovascular Disease Prevention in Adults with Cardiovascular Risk Factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(20):2069–75. [DOI] [PubMed] [Google Scholar]
- 8.Rosato V, Temple NJ, La Vecchia C, Castellan G, Tavani A, Guercio V. Mediterranean diet and cardiovascular disease: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2019;58(1):173–91. [DOI] [PubMed] [Google Scholar]
- 9.Ramezani-Jolfaie N, Mohammadi M, Salehi-Abargouei A. The effect of healthy Nordic diet on cardio-metabolic markers: a systematic review and meta-analysis of randomized controlled clinical trials. Eur J Nutr. 2019;58(6):2159–74. [DOI] [PubMed] [Google Scholar]
- 10.Chiavaroli L, Viguiliouk E, Nishi S, Blanco Mejia S, Rahelić D, Kahleová H, et al. DASH Dietary Pattern and Cardiometabolic Outcomes: an Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients. 2019;11(2):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belli M, Barone L, Longo S, Prandi FR, Lecis D, Mollace R, et al. Gut Microbiota Composition and Cardiovascular Disease: a Potential New Therapeutic Target? Int J Mol Sci. 2023;24(15):11971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nesci A, Carnuccio C, Ruggieri V, D’Alessandro A, Di Giorgio A, Santoro L, et al. Gut Microbiota and Cardiovascular Disease: Evidence on the Metabolic and Inflammatory Background of a Complex Relationship. Int J Mol Sci. 2023;24(10):9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazemian N, Mahmoudi M, Halperin F, Wu JC, Pakpour S. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome, 2020;8(1):36. [DOI] [PMC free article] [PubMed]
- 14.Perler BK, Friedman ES, Wu GD. The Role of the Gut Microbiota in the Relationship Between Diet and Human Health. Annu Rev Physiol. 2023;85(1):449–68. [DOI] [PubMed] [Google Scholar]
- 15.Rinott E, Meir AY, Tsaban G, Zelicha H, Kaplan A, Knights D, et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: a randomized controlled trial. Genome Med. 2022;14(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meslier V, Laiola M, Roager HM, De Filippis F, Roume H, Quinquis B, et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut. 2020;69(7):1258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos-Marcos JA, Haro C, Vega-Rojas A, Alcala-Diaz JF, Molina-Abril H, Leon-Acuña A, et al. Sex Differences in the Gut Microbiota as Potential Determinants of Gender Predisposition to Disease. Mol Nutr Food Res. 2019;63(7):e1800870. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69(7):1218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Iorio BR, Rocchetti MT, De Angelis M, Cosola C, Marzocco S, Di Micco L, et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Serum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study). J Clin Med. 2019;8(9). [DOI] [PMC free article] [PubMed]
- 20.Aromataris E, Lockwood C, Porritt K, Pilla B, Jordan Z, editors. JBI Manual for Evidence Synthesis. JBI; 2024. Available from https://synthesismanual.jbi.global. 10.46658/JBIMES-24-01.
- 21.Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne J, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 23.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dersimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–14. [DOI] [PubMed] [Google Scholar]
- 25.Schünemann HJ, Brozek J, Guyatt G, Oxman AD. GRADE handbook: introduction to GRADE handbook. 2013. https://gdt.gradepro.org/app/handbook/handbook.html#h.w6r7mtvq3mjz.
- 26.Guevara-Cruz M, Flores-López AG, Aguilar-López M, Sánchez-Tapia M, Medina-Vera I, Díaz D, et al. Improvement of Lipoprotein Profile and Metabolic Endotoxemia by a Lifestyle Intervention that Modifies the Gut Microbiota in Subjects with Metabolic Syndrome. J Am Heart Assoc. 2019;8(17):e012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Y, Luo S, Ye Y, Yin S, Fan J, Xia M. Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients. J Clin Endocrinol Metab. 2021;106(1):64–79. [DOI] [PubMed] [Google Scholar]
- 28.Haro C, Garcia-Carpintero S, Alcala-Diaz JF, Gomez-Delgado F, Delgado-Lista J, Perez-Martinez P, et al. The gut microbial community in metabolic syndrome patients is modified by diet. J Nutr Biochem. 2016;27:27–31. [DOI] [PubMed] [Google Scholar]
- 29.Haro C, García-Carpintero S, Rangel-Zúñiga OA, Alcalá-Díaz JF, Landa BB, Clemente JC, et al. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol Nutr Food Res. 2017;61(12):1700300. [DOI] [PubMed]
- 30.Haro C, Montes-Borrego M, Rangel-Zúñiga OA, Alcalá-Díaz JF, Gómez-Delgado F, Pérez-Martínez P, et al. Two Healthy Diets Modulate Gut Microbial Community Improving Insulin Sensitivity in a Human Obese Population. J Clin Endocrinol Metab. 2016;101(1):233–42. [DOI] [PubMed] [Google Scholar]
- 31.Maifeld A, Bartolomaeus H, Löber U, Avery EG, Steckhan N, Markó L, et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat Commun. 2021;12(1):1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muralidharan J, Moreno-Indias I, Bulló M, Lopez JV, Corella D, Castañer O, et al. Effect on gut microbiota of a 1-y lifestyle intervention with Mediterranean diet compared with energy-reduced Mediterranean diet and physical activity promotion: PREDIMED-Plus Study. Am J Clin Nutr. 2021;114(3):1148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Trijp MPH, Schutte S, Esser D, Wopereis S, Hoevenaars FPM, Hooiveld GJEJ, et al. Minor Changes in the Composition and Function of the Gut Microbiota During a 12-Week Whole Grain Wheat or Refined Wheat Intervention Correlate with Liver Fat in Overweight and Obese Adults. J Nutr. 2021;151(3):491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vetrani C, Maukonen J, Bozzetto L, Della PG, Vitale M, Costabile G, et al. Diets naturally rich in polyphenols and/or long-chain n-3 polyunsaturated fatty acids differently affect microbiota composition in high-cardiometabolic-risk individuals. Acta Diabetol. 2020;57(7):853–60. [DOI] [PubMed] [Google Scholar]
- 35.Choo JM, Murphy KJ, Wade AT, Wang Y, Bracci EL, Davis CR, et al. Interactions between Mediterranean Diet Supplemented with Dairy Foods and the Gut Microbiota Influence Cardiovascular Health in an Australian Population. Nutrients. 2023;15(16):3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Djekic D, Shi L, Brolin H, Carlsson F, Särnqvist C, Savolainen O, et al. Effects of a Vegetarian Diet on Cardiometabolic Risk Factors, Gut Microbiota, and Plasma Metabolome in Subjects with Ischemic Heart Disease: a Randomized, Crossover Study. J Am Heart Assoc. 2020;9(18):e016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eriksen AK, Brunius C, Mazidi M, Hellström PM, Risérus U, Iversen KN, et al. Effects of whole-grain wheat, rye, and lignan supplementation on cardiometabolic risk factors in men with metabolic syndrome: a randomized crossover trial. Am J Clin Nutr. 2020;111(4):864–76. [DOI] [PubMed] [Google Scholar]
- 38.Galié S, García-Gavilán J, Camacho-Barcía L, Atzeni A, Muralidharan J, Papandreou C, et al. Effects of the Mediterranean Diet or Nut Consumption on Gut Microbiota Composition and Fecal Metabolites and their Relationship with Cardiometabolic Risk Factors. Mol Nutr Food Res. 2021;65(19):e2000982. [DOI] [PubMed] [Google Scholar]
- 39.Hald S, Schioldan AG, Moore ME, Dige A, Laerke HN, Agnholt J, et al. Effects of Arabinoxylan and Resistant Starch on Intestinal Microbiota and Short-Chain Fatty Acids in Subjects with Metabolic Syndrome: a Randomised Crossover Study. PLoS ONE. 2016;11(7):e0159223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagliai G, Russo E, Niccolai E, Dinu M, Di Pilato V, Magrini A, et al. Influence of a 3-month low-calorie Mediterranean diet compared to the vegetarian diet on human gut microbiota and SCFA: the CARDIVEG Study. Eur J Nutr. 2020;59(5):2011–24. [DOI] [PubMed] [Google Scholar]
- 41.Petersen KS, Anderson S, Chen See JR, Leister J, Kris-Etherton PM, Lamendella R. Herbs and Spices Modulate Gut Bacterial Composition in Adults at Risk for CVD: Results of a Prespecified Exploratory Analysis from a Randomized, Crossover, Controlled-Feeding Study. J Nutr. 2022;152(11):2461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roager HM, Vogt JK, Kristensen M, Hansen L, Ibrügger S, Mærkedahl RB, et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut. 2019;68(1):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahrens AP, Culpepper T, Saldivar B, Anton S, Stoll S, Handberg EM, et al. A Six-Day, Lifestyle-Based Immersion Program Mitigates Cardiovascular Risk Factors and Induces Shifts in Gut Microbiota, Specifically Lachnospiraceae, Ruminococcaceae, Faecalibacterium prausnitzii: a Pilot Study. Nutrients. 2021;13(10):3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark RL, Famodu OA, Holásková I, Infante AM, Murray PJ, Olfert IM, et al. Educational intervention improves fruit and vegetable intake in young adults with metabolic syndrome components. Nutr Res. 2019;62:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahleova H, Rembert E, Alwarith J, Yonas WN, Tura A, Holubkov R, et al. Effects of a Low-Fat Vegan Diet on Gut Microbiota in Overweight Individuals and Relationships with Body Weight, Body Composition, and Insulin Sensitivity. A Randomized Clinical Trial. Nutrients. 2020;12(10):2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barber TM, Kabisch S, Pfeiffer AFH, Weickert MO. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients. 2023;15(9):2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen R, Zhang C, Xu F, Yu L, Tian F, Chen W, et al. Meta-analysis reveals gut microbiome and functional pathway alterations in response to resistant starch. Food Funct. 2023;14(11):5251–63. [DOI] [PubMed] [Google Scholar]
- 48.Kimble R, Gouinguenet P, Ashor A, Stewart C, Deighton K, Matu J, et al. Effects of a mediterranean diet on the gut microbiota and microbial metabolites: a systematic review of randomized controlled trials and observational studies. Crit Rev Food Sci Nutr. 2023;63(27):8698–719. [DOI] [PubMed] [Google Scholar]
- 49.Hu X, Xia K, Dai M, Han X, Yuan P, Liu J, et al. Intermittent fasting modulates the intestinal microbiota and improves obesity and host energy metabolism. NPJ Biofilms Microbiomes. 2023;9(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gorvitovskaia A, Holmes SP, Huse SM. Interpreting Prevotella and Bacteroides as biomarkers of diet and lifestyle. Microbiome. 2016;4(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, et al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014;15(7):R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biddle A, Stewart L, Blanchard J, Leschine S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity. 2013;5(3):627–40. [Google Scholar]
- 53.Martin R, Bermudez-Humaran LG, Langella P. Searching for the Bacterial Effector: the Example of the Multi-Skilled Commensal Bacterium Faecalibacterium prausnitzii. Front Microbiol. 2018;9:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu T, Wu Q, Yao Q, Jiang K, Yu J, Tang Q. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res Rev. 2022;81:101706. [DOI] [PubMed] [Google Scholar]
- 55.Fusco W, Lorenzo MB, Cintoni M, Porcari S, Rinninella E, Kaitsas F, et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients. 2023;15(9):2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Jiang Q, Liu Z, Shen S, Ai J, Zhu Y, et al. Alteration of Gut Microbiota Relates to Metabolic Disorders in Primary Aldosteronism Patients. Front Endocrinol (Lausanne). 2021;12:667951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoo JY, Sniffen S, Mcgill Percy KC, Pallaval VB, Chidipi B. Gut Dysbiosis and Immune System in Atherosclerotic Cardiovascular Disease (ACVD). Microorganisms. 2022;10(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bultman SJ. Bacterial butyrate prevents atherosclerosis. Nat Microbiol. 2018;3(12):1332–3. [DOI] [PubMed] [Google Scholar]
- 59.Chen W, Zhang S, Wu J, Ye T, Wang S, Wang P, et al. Butyrate-producing bacteria and the gut-heart axis in atherosclerosis. Clin Chim Acta. 2020;507:236–41. [DOI] [PubMed] [Google Scholar]
- 60.Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms. 2020;8(4):573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patikorn C, Roubal K, Veettil SK, Chandran V, Pham T, Lee YY, et al. Intermittent Fasting and Obesity-Related Health Outcomes: an Umbrella Review of Meta-analyses of Randomized Clinical Trials. JAMA Netw Open. 2021;4(12):e2139558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ge L, Sadeghirad B, Ball G, Da CB, Hitchcock CL, Svendrovski A, et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. BMJ. 2020;369:m696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vadiveloo M, Parker H, Raynor H. Increasing low-energy-dense foods and decreasing high-energy-dense foods differently influence weight loss trial outcomes. Int J Obes (Lond). 2018;42(3):479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Izadi V, Haghighatdoost F, Moosavian P, Azadbakht L. Effect of Low-Energy-Dense Diet Rich in Multiple Functional Foods on Weight-Loss Maintenance, Inflammation, and Cardiovascular Risk Factors: a randomized controlled trial. J Am Coll Nutr. 2018;37(5):399–405. [DOI] [PubMed] [Google Scholar]
- 65.Schwingshackl L, Hoffmann G. Comparison of effects of long-term low-fat vs high-fat diets on blood lipid levels in overweight or obese patients: a systematic review and meta-analysis. J Acad Nutr Diet. 2013;113(12):1640–61. [DOI] [PubMed] [Google Scholar]
- 66.Chawla S, Tessarolo SF, Amaral MS, Mekary RA, Radenkovic D. The Effect of Low-Fat and Low-Carbohydrate Diets on Weight Loss and Lipid Levels: a Systematic Review and Meta-Analysis. Nutrients. 2020;12(12):3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaiser KA, Brown AW, Bohan Brown MM, Shikany JM, Mattes RD, Allison DB. Increased fruit and vegetable intake has no discernible effect on weight loss: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100(2):567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bendall CL, Mayr HL, Opie RS, Bes-Rastrollo M, Itsiopoulos C, Thomas CJ. Central obesity and the Mediterranean diet: a systematic review of intervention trials. Crit Rev Food Sci Nutr. 2018;58(18):3070–84. [DOI] [PubMed] [Google Scholar]
- 69.Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Fitó M, Chiva-Blanch G, et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: a prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):e6–17. [DOI] [PubMed] [Google Scholar]
- 70.Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, et al. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J Am Coll Cardiol. 2017;70(4):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arabzadegan N, Daneshzad E, Fatahi S, Moosavian SP, Surkan PJ, Azadbakht L. Effects of dietary whole grain, fruit, and vegetables on weight and inflammatory biomarkers in overweight and obese women. Eat Weight Disord. 2020;25(5):1243–51. [DOI] [PubMed] [Google Scholar]
- 72.Estruch R, Ros E. The role of the Mediterranean diet on weight loss and obesity-related diseases. Rev Endocr Metab Disord. 2020;21(3):315–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.