Abstract
Cytosolic free Ca2+ plays an important role in the molecular mechanisms leading to regulated insulin secretion by the pancreatic β cell. A number of Ca2+-binding proteins have been implicated in this process. Here, we define the role of the Ca2+-binding protein neuronal Ca2+ sensor-1 (NCS-1) in insulin secretion. In pancreatic β cells, NCS-1 increases exocytosis by promoting the priming of secretory granules for release and increasing the number of granules residing in the readily releasable pool. The effect of NCS-1 on exocytosis is mediated through an increase in phosphatidylinositol (PI) 4-kinase β activity and the generation of phosphoinositides, specifically PI 4-phosphate and PI 4,5-bisphosphate. In turn, PI 4,5-bisphosphate controls exocytosis through the Ca2+-dependent activator protein for secretion present in β cells. Our results provide evidence for an essential role of phosphoinositide synthesis in the regulation of glucose-induced insulin secretion by the pancreatic β cell. We also demonstrate that NCS-1 and its downstream target, PI 4-kinase β, are critical players in this process by virtue of their capacity to regulate the release competence of the secretory granules.
Keywords: insulin, phosphoinositides, islet, secretion, Ca2+-dependent activator protein for secretion
Neuronal Ca2+ sensor-1 (NCS-1) belongs to the family of EF-hand Ca2+-binding proteins and is mainly expressed in neuronal and neuroendocrine cells, where it enhances neurotransmission and Ca2+-dependent exocytosis (1-5). NCS-1 interacts with and regulates the activity of phosphatidylinositol 4-kinase β (PI4Kβ) (6-9). The members of the PI4K family catalyze the first step in the synthesis of PI 4,5-bisphosphate [PI(4,5)P2], which has recently emerged as an important regulator of Ca2+-dependent secretion (10-12). Both a PI transfer protein (13) and a PI4P 5-kinase (14) are required for regulated exocytosis of dense core granules. In addition, the presence of synaptic vesicle and dense core granule-associated PI4K activity is essential for exocytosis (15, 16). These data imply that generation of PI(4,5)P2 is an important step in the event of secretion.
In pancreatic β cells, glucose dose-dependently increases ATP levels and decreases ADP levels (16). The resulting rise in the ATP at the expense of ADP is an important regulator of the two major signaling pathways involved in glucose-induced insulin secretion. The first of these pathways uses ATP-sensitive K+ channels to couple glucose metabolism with electrical activity, Ca2+ influx, and initiation of insulin secretion. The second pathway is exerted at the level of granule priming and regulates the β cell secretory capacity by modulation of the granules' release competence (17). The β cell contains ≈10,000 insulin-containing secretory granules (18). Interestingly, as many as ≈5% of the granules are docked below the membrane. The readily releasable pool (RRP), defined by functional measurements, represents a subset (50-100 granules) of the docked pool (19). Granules belonging to the RRP are immediately available for release. The majority of the granules belong to the nonreleasable pool (reserve pool) that must undergo a series of ATP-, Ca2+-, and time-dependent reactions (referred to as mobilization or priming) to gain release competence (19). After the depletion of the RRP, this pool is replenished by mobilization of new granules from the reserve pool. The number of granules residing in the docked pool is sufficient for several hours of glucose-induced insulin secretion (19). This observation suggests that mobilization does not require physical translocation of the secretory granules and that mobilization of granules already in place may be sufficient to account for the refilling of the RRP.
We have recently demonstrated that in β cells, a PI4K serves as a metabolic sensor and, through generation of PI(4,5)P2, regulates ATP-dependent priming of secretory granules (20). PI(4,5)P2 binds specifically to the Ca2+-dependent activator protein for secretion (CAPS), which is required for Ca2+-triggered exocytosis once ATP-dependent priming has been completed (20-23). In the present study, we have explored the signaling mechanisms by which glucose stimulates PI4K activity in the pancreatic β cell. We demonstrate that NCS-1 acts as a Ca2+ sensor in β cells and promotes priming of secretory granules through regulation of PI4Kβ activity. The interplay between NCS-1 and PI4Kβ represents a molecular mechanism by which Ca2+ and metabolic signaling of the β cell are integrated to finely adjust insulin secretion to ambient glucose concentrations.
Materials and Methods
Preparation and Culture of β Cells. Mouse pancreatic islets were isolated from female NMRI mice (Bomholtgaard, Ry, Denmark) as described in ref. 24. Briefly, the mice were stunned by a blow against the head and killed by cervical dislocation as approved by the local ethical committees. The pancreas was quickly removed, and islets of Langerhans were isolated by collagenase (Sigma-Aldrich) digestion. Single islet cells were prepared by shaking in a Ca2+-free solution. Cells were incubated in RPMI medium 1640 (GIBCO/BRL) supplemented with 10% (vol/vol) heat-inactivated FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin. INS-1E cells were cultured as described in ref. 25.
Expression Vectors and Transfection. The generation of the bicistronic expression construct encoding rat NCS-1 in pIRES2-EGFP (BD Biosciences Clontech) was described in refs. 1 and 7. The human PI4Kβ wild-type and dominant-negative kinase-dead D656A cDNA constructs were kindly provided by Rachel Meyers and were described in refs. 26 and 27. All expression constructs were analyzed by DNA sequencing using appropriate primers and a cycle sequencing kit (BigDye Terminator, Version 3.1, Applied Biosystems). Single mouse islet cells and INS-1E cells were transfected on the day after plating with pIRES2-EGFP (mock) or construct of interest at 2 μg/ml in cell culture medium by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Lipofectamine was used in a ratio of 4:1 (vol/wt) to DNA. INS-1E cells were plated out in a density to obtain ≈90% confluency on day 2 after transfection, when cells were used for studies. Based on GFP fluorescence, the transfection efficiency amounted to 7 ± 2% (n = 744 cells; seven different cell preparations and transfections) in mouse islet cells and 57 ± 4% (n = 1,320 cells; eight different transfections) in INS-1E cells. Western blot analysis revealed that the level of overexpression for the different constructs was >5-fold.
Cell Fractionation and Immunoblotting. INS-1E cells were homogenized in a buffer containing 20 mM Hepes, 1 mM MgCl2, 1 mM EGTA, and 250 mM sucrose (pH 7.4) and supplemented with protease inhibitor mixture (Roche Diagnostics). Homogenate was centrifuged at 1,000 × g for 10 min and supernatant was collected and applied on the top of a discontinuous sucrose gradient (0.6-1.8 M sucrose). Samples were centrifuged in a swinging-bucket rotor at 110,000 × g for 16 h at 4°C. After centrifugation fractions were collected and protein concentration in each fraction was measured, 20 μg of protein from each fraction was applied to SDS/PAGE. Proteins were separated and transferred to poly(vinylidene difluoride) membrane, and immunoblotting was performed. Antibodies against the following proteins were used: glucokinase and NCS-1 (Santa Cruz Biotechnology) and chromogranin A, PI4Kβ, VLA-2α, GM130, and GRP78 (BD Biosciences Pharmingen).
Capacitance Measurements. Two days after transfection, cells expressing EGFP were selected for capacitance measurements. Exocytosis was monitored as changes in cell capacitance by using either the perforated-patch or standard whole-cell configuration of the patch-clamp technique. For standard whole-cell experiments, the pipette solution contained 125 mM cesium glutamate, 10 mM CsCl, 10 mM NaCl, 1 mM MgCl2, 5 mM Hepes, 0.05 mM EGTA, 3 mM MgATP, and 0.01 mM GTP (pH 7.15 with CsOH). The capacitance measurements commenced 2 min after establishment of the whole-cell configuration to allow equilibration between the pipette solution and the cytoplasm. In perforated-patch experiments, the pipette solution consisted of 76 mM Cs2SO4, 10 mM NaCl, 10 mM KCl, 1 mM MgCl2, 5 mM Hepes (pH 7.35 with CsOH), and 0.24 mg/ml amphotericin B. The extracellular medium consisted of 118 mM NaCl, 20 mM tetraethylammonium chloride, 5.6 mM KCl, 1.2 mM MgCl2, 2.6 mM CaCl2, 5 mM Hepes (pH 7.40 with NaOH), and 3 or 20 mM glucose. The stimulation protocol consisted of trains of 10 500-ms depolarizations applied at 1 Hz and went from -70 to 0 mV. The capacitance measurements were performed at 33°C.
PI4K Assay. INS-1E cells transfected with plasmids of interest were seeded in 48-well plates (2 × 105 cells per well). After being cultured for 48 h, the cells were incubated for 1 h in extracellular medium containing either 3 or 20 mM glucose. Cells were harvested in 400 μl of 25 mM N-[tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid (TES) buffer (pH 6.90) containing 5 mM MgCl2, 1 mM EGTA, 0.1 mM DTT, and 10 mM benzamidine. A homogenate was prepared by sonication (10 s, 40 W) and centrifuged at 100,000 × g (60 min at 4°C). The particulate fraction was solubilized in 400 μl of the same buffer containing 0.125% (vol/vol) Triton X-100 by incubation for 60 min at 4°C. Samples were incubated for 10 min at 30°C in a reaction medium (50 μl) containing 35 mM TES buffer (pH 6.90), 6 mM MgCl2, 1.2 mM EGTA, 0.12 mM DTT, 1 mg/ml phosphoinositide mixture (Sigma), and 50 μM [γ-32P]ATP (≈3,000 cpm/pmol). The reaction was terminated by the addition of 400 μl of 1 M HCl. Lipids were extracted and separated as described in ref. 20.
Assays of Phosphoinositides. INS-1E cells transfected with plasmids of interest were seeded in 48-well plates (2 × 105 cells per well). After culture for 48 h in RPMI medium 1640 supplemented with myo-[3H]inositol (4 μCi/ml; 1 Ci = 37 GBq), cells were equilibrated for 1 h in extracellular medium containing 3 mM glucose and 10 mM LiCl before incubation for 1 h in medium containing 3 or 20 mM glucose. Incubations were terminated by the addition of an equal volume of 1 M trichloroacetic acid. The supernatant was removed, and cell residues were extracted with acidified chloroform/methanol. Deacylation and separation of glycerophosphoinositol was performed (28).
Human Growth Hormone (hGH) Release Assay. After transfection with pCMV5-hGH (29) and either empty vector pcDNA3 or plasmid of interest, INS-1E cells were seeded in 48-well plates (2 × 105 cells per well) and cultured for 48 h. Incubation and secretion experiments were performed as described in ref. 29. hGH levels in the various samples were measured by using ELISA (Roche).
Immunohistochemistry. Fourteen-micrometer sections from rat pancreas were prepared and processed for immunofluorescence histochemistry as described in ref. 30. Sections were double-labeled with rabbit antiserum to NCS-1 (diluted 1:300) and mouse monoclonal antibodies to insulin (diluted 1:600,000; Sigma). Chicken antiserum to NCS-1 (diluted 1:15; ref. 1) was combined with rabbit antiserum to PI4Kβ (diluted 1:400; generated as described in ref. 27). Costaining of pancreatic islets with chicken and rabbit antibodies to NCS-1 showed similar localization (data not shown). The primary antibodies were visualized by using a mixture of Cy5-conjugated donkey anti-rabbit (diluted 1:250) and FITC-conjugated donkey anti-mouse (diluted 1:80) or Cy5-conjugated donkey anti-chicken (diluted 1:250) and FITC-conjugated donkey anti-rabbit (diluted 1:80) secondary antibodies. All secondary antibodies were from Jackson ImmunoResearch.
Statistical Analysis. Results are presented as mean values ± SEM for the indicated number of experiments. Statistical significances were evaluated by using Student's t test for paired data or Dunnett's test for multiple comparisons.
Results and Discussion
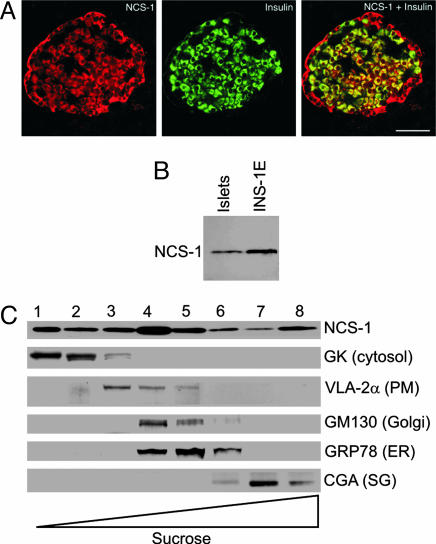
Double-labeling immunofluorescence histochemistry of pancreatic islets demonstrated NCS-1 immunoreactivity in all pancreatic islet cells, including insulin-secreting β cells (Fig. 1A). Immunoblotting confirmed that the same antibody recognized a single immunoreactive band (≈21 kDa) in NMRI mouse islets and insulin-secreting INS-1E cells (Fig. 1B). Subcellular fractionation studies of INS-1E cells demonstrated that NCS-1 is present in all fractions with strong signal in the cytosolic-, endoplasmic reticulum-, Golgi-, and secretory granule-containing fractions (Fig. 1C). These observations are in line with previous data from rat brain (8) as well as PC12 and chromaffin cells (31, 32), indicating that NCS-1 has a broad cellular distribution in secretory cells.
Fig. 1.
NCS-1 immunoreactivity is present in pancreatic β cells. (A) Representative image of a section of rat pancreatic islet immunostained with antisera to NCS-1 (Left) and insulin (Center) and their overlay (Right). (Scale bar: 50 μm.) (B) Western blot showing presence of NCS-1 protein in NMRI mouse islets and INS-1E cells. (C) Subcellular localization of NCS-1 in INS-1E cells. Subcellular fractions were obtained by sucrose density gradient centrifugation. NCS-1 and organelle markers were detected by using specific antibodies. The experiment was repeated three times with similar results. GK, glucokinase; PM, plasma membrane; ER, endoplasmic reticulum; CGA, chromogranin A; SG, secretory granule.
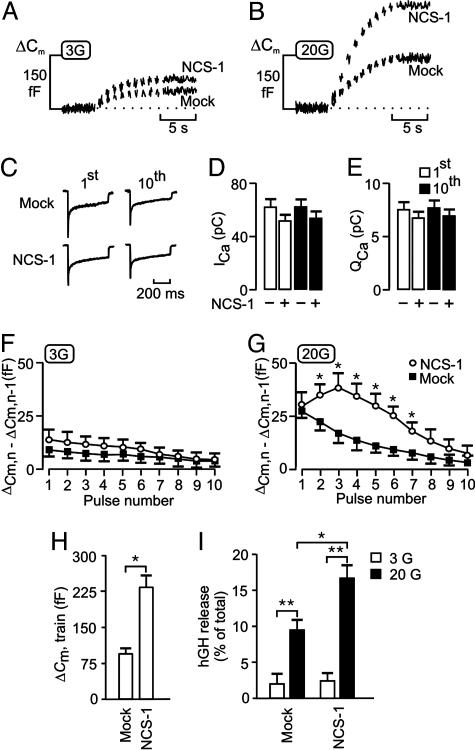
Having established that NCS-1 is present in β cells, we explored its role in Ca2+- and glucose-dependent exocytosis. Fig. 2A illustrates the exocytotic responses (measured as increases in cell capacitance) elicited by a train of 10 500-ms voltage-clamp depolarizations from -70 to 0 mV in mouse β cells in the presence of 3 mM glucose. In cells transfected with EGFP (mock), there was a progressive decline in the amplitude of the exocytotic responses during the train until eventually, after the sixth pulse, depolarization failed to produce any further increase in cell capacitance (Fig. 2F). A similar pattern of exocytotic response was observed in nontransfected β cells (data not shown, but see ref. 20). As mentioned above, the secretory granules in the β cell can functionally be divided into the RRP and a reserve pool. The depression in exocytotic capacity that we attribute to the depletion of the RRP and the capacitance ceases to increase once this pool is empty. In a series of five experiments, the total capacitance increase evoked by the train averaged 49 ± 7 fF. Using a conversion factor of 3 fF per granule (18), the RRP can be estimated to contain 15-20 granules at the onset of the train. Cessation of exocytosis was not attributable to inactivation of the Ca2+ current, because the peak and integrated Ca2+ currents measured at the end of the train were only marginally reduced relative to the initial amplitude (Fig. 2 C-E). In β cells transfected with NCS-1, the magnitude of the exocytotic responses evoked by the entire train increased marginally (30-40% over the control level; Fig. 2 A and F), an effect that was not associated with an increase in the magnitude of the peak or integrated whole-cell Ca2+ current (Fig. 2 C-E). Interestingly, NCS-1 has been reported to enhance (33), inhibit (34), or not alter (5) Ca2+ channel activity in different secretory cell types, and the β cell fits in the latter category.
Fig. 2.
NCS-1 facilitates glucose- and Ca2+-dependent exocytosis. Individual mouse β cells were transfected with EGFP (mock) or NCS-1 and subjected to a train of 10 500-ms depolarizations by using the perforated-patch configuration. Increases in cell capacitance (ΔCm) were measured at 3 mM (A) and 20 mM (B) glucose. (C) Whole-cell Ca2+ currents evoked by the first (1st) and last (10th) depolarization of the trains giving rise to the capacitance increases depicted in A in mock- and NCS-1-transfected cells. (D and E) Histograms summarizing the effects of NCS-1 overexpression in mouse β cells on the peak (D) and integrated (E) whole-cell Ca2+ currents evoked by the first (1st) and last (10th) pulse of a train of 10 depolarizations. The cells were exposed to 3 mM glucose. (F and G) Summary data of amplitudes of increases in cell capacitance for each depolarizing pulse (1-10) in the presence of 3 mM (F) or 20 mM (G) glucose in mock-transfected cells (▪) or cells transfected with NCS-1 (○). (H) Histogram summarizing the magnitude of the exocytotic responses elicited by trains of depolarizations (ΔCm, train) in cells transfected with EGFP (mock) or NCS-1. The experiments were performed in the presence of 20 mM glucose. In D-H, data are quoted as mean values ± SEM of five to seven experiments in each group. (I) INS-1E cells were cotransfected in parallel with pCMV5-hGH and empty vector (pcDNA3) (mock) or with pCMV5-hGH and NCS-1. hGH secretion was measured in Krebs-Ringer bicarbonate Hepes buffer with 3 mM (□)or 20 mM (▪) glucose. hGH release is depicted as secreted hGH in percentage of total hGH. Values are mean ± SEM of three experiments (each in triplicate). *, P < 0.05; **, P < 0.01.
We repeated the same experimental protocol in cells exposed to 20 mM glucose. In agreement with results described in ref. 35, glucose stimulation strongly enhanced the capacitance increase in response to the train of depolarizations (Fig. 2B). The total increase in cell capacitance in mock-transfected cells averaged 49 ± 7 fF and 88 ± 10 fF (P < 0.01; n = 5) in the presence of 3 and 20 mM glucose, respectively. This stimulation of exocytosis occurred without an increase in the whole-cell Ca2+ current (data not shown, but see Fig. 5C). Close inspection of the time course of exocytosis revealed that glucose augmented the capacitance increases mainly during the first four membrane depolarizations (Fig. 2 F and G). However, despite this stimulation of exocytosis, a progressive decline in the amplitude of the exocytotic responses during the train was observed (Fig. 2 B and G). NCS-1-transfected cells showed a strong and consistent facilitation of exocytosis in the presence of 20 mM glucose. This switch from depression to facilitation was most evident during the second to sixth pulse and was consistently absent from the first pulse (Fig. 2G). An explanation for this could be that, as the cytoplasmic Ca2+ concentration ([Ca2+]i) builds up during the train of depolarizations, NCS-1 is activated, which results in facilitation of release. The capacitance increases evoked by the entire train was increased from 88 ± 10 fF (n = 5) in mock-transfected cells to 230 ± 14 fF (P < 0.01; n = 5) in cells transfected with NCS-1 (Fig. 2H). We conclude that NCS-1 positively regulates the pathway leading to Ca2+-dependent exocytosis in β cells by promoting the priming of secretory granules in the reserve pool for release and thereby increasing the number of granules residing in the RRP.
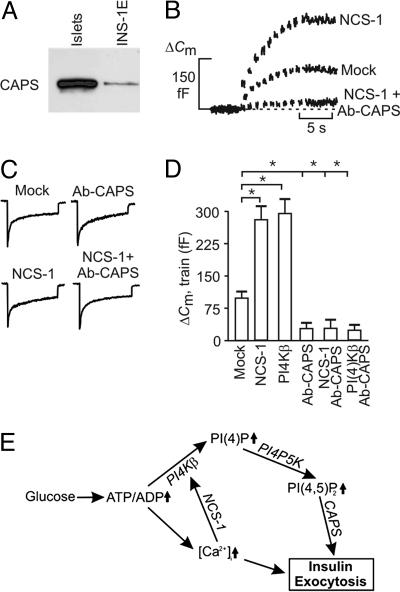
Fig. 5.
CAPS is involved in NCS-1- and PI4Kβ-dependent exocytosis. (A) Western blot showing expression of CAPS in NMRI mouse islets and INS-1E cells. (B) Capacitance increases elicited by trains of 10 500-ms depolarizations in single mouse β cells transfected with EGFP (mock) or NCS-1. The experiments commenced 2 min after establishment of the standard whole-cell configuration. Increases in cell capacitance also were recorded in cells expressing NCS-1 after inclusion of an antibody against CAPS (anti-CAPS, 1.5 mg/ml) in the pipette-filling solution (NCS-1 plus Ab-CAPS). All experiments were conducted in the presence of 20 mM glucose. (C) Whole-cell Ca2+ currents evoked by the first pulse in a train of membrane depolarizations in mock- and NCS-1-transfected cells in the absence and presence of antibody against CAPS (Ab-CAPS). (D) Histogram summarizing exocytosis elicited by trains of depolarizations applied 2 min after establishment of the whole-cell configuration in cells transfected with EGFP (mock), NCS-1, or PI4Kβ in the absence and presence of an antibody against CAPS (Ab-CAPS, 1.5 mg/ml). Data are mean values ± SEM of five to seven experiments. *, P < 0.01. (E) Model for regulation of exocytosis by NCS-1 and PI4Kβ after glucose stimulation in pancreatic β cells. High glucose raises the ATP/ADP ratio. Increased ATP/ADP leads to membrane depolarization and, ultimately, to an increase in cytoplasmic Ca2+ concentration, [Ca2+]i. Both elevated ATP/ADP (directly) and [Ca2+]i (through NCS-1) stimulate PI4Kβ activity. PI(4)P and PI(4,5)P2, formed upon the sequential stimulation of PI4Kβ and PI4P 5-kinase, enhance Ca2+-induced insulin exocytosis.
To confirm a role of NCS-1 in the control of exocytosis, we tested the effect of its overexpression in INS-1E cells by using the hGH transient cotransfection assay in which hGH acts as a reporter of exocytosis from transfected cells only. INS-1E cells represent a suitable cell system, because total increases in cell capacitance in cells overexpressing NCS-1 were comparable with those observed in primary mouse β cells (data not shown). In control INS-1E cells (cotransfected in parallel with pCMV5-hGH and empty vector pcDNA3), there was a significant stimulation of hGH secretion (P < 0.05; n = 5) after the addition of 20 mM glucose, compared with 3 mM glucose (Fig. 2I). Over-expression of NCS-1 had no effect on basal hGH secretion but revealed a significant increase in the presence of 20 mM glucose.
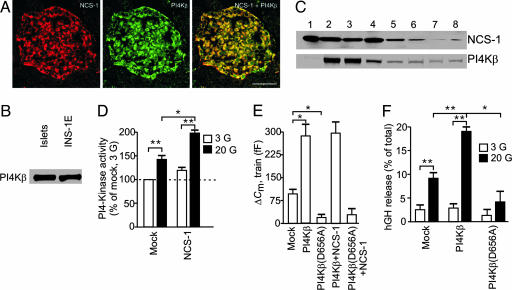
In neuroendocrine cells, PI4Kβ is a downstream target of NCS-1 in enhancing exocytosis. NCS-1 interacts with PI4Kβ and stimulates kinase activity. This finding is supported by colocalization of NCS-1 and PI4Kβ immunoreactivity in virtually all pancreatic islet cells (Fig. 3A). Immunoblotting confirmed that the same antibody to PI4Kβ recognized a single band (90 kDa) in NMRI mouse islets and INS-1E cells (Fig. 3B). Subcellular fractionation studies in INS-1E cells revealed that PI4Kβ has a broad distribution and that the majority of the protein is present in fractions 2-4, likely resembling both soluble material and some light membranous organelles. A comparison of Figs. 1C and 3C suggests that PI4Kβ colocalizes with NCS-1 in the secretory granule-, endoplasmic reticulum-, and Golgi-containing fractions (Fig. 3C).
Fig. 3.
PI4Kβ is a downstream target of NCS-1 and stimulates exocytosis in β cells. (A) Images of a rat pancreatic islet immunostained with antisera to NCS-1 (Left) and PI4Kβ (Center) and their overlay (Right). (Scale bar: 50 μm.) (B) Western blot showing expression of PI4Kβ in NMRI mouse islets and INS-1E cells. (C) NCS-1 and PI4Kβ subcellular distribution in fractions obtained by sucrose density gradient centrifugation of INS-1E cells (for fraction characterization, see Fig. 1C). The experiments were repeated three times with similar results. (D) PI4K activity measured in homogenates from INS-1E cells transfected with EGFP (mock) or NCS-1 in the presence of either 3 mM (□) or 20 mM (▪) glucose. Data are expressed relative to those determined in mock-transfected cells at 3 mM glucose and represent mean ± SEM of three different experiments (each in duplicate). (E) Histogram summarizing the magnitude of the exocytotic responses elicited by trains of depolarizations (ΔCm, train) in mouse β cells transfected with EGFP (mock) and either PI4Kβ or PI4Kβ(D656A) alone or with NCS-1. The experiments were performed at 20 mM glucose and are quoted as mean values ± SEM of five to eight experiments in each group. (F) INS-1E cells were cotransfected in parallel with pCMV5-hGH and empty vector (pcDNA3) (mock) or with pCMV5-hGH and either PI4Kβ or PI4Kβ(D656A). hGH secretion was measured at 3 mM (□) or 20 mM (▪) glucose. hGH release is depicted as secreted hGH in percentage of total hGH. Values are mean ± SEM of three experiments (each in triplicate). *, P < 0.01; **, P < 0.005.
Next, we investigated the effects of glucose and NCS-1 on PI4K activity in INS-1E cells. Increasing the glucose concentration from 3 to 20 mM produced a 42 ± 6% (P < 0.01) stimulation of PI4K activity (Fig. 3D). Overexpression of NCS-1 significantly stimulated PI4K activity at high glucose concentration (Fig. 3D).
To explore the role of PI4Kβ in the recruitment of granules for release, we recorded increases in cell capacitance in response to a train of depolarizations. Fig. 3E illustrates average increases in exocytosis in mock- and PI4Kβ-transfected mouse β cells. It is clear that the capacitance increases evoked by the train of depolarizations were augmented by 2-fold in cells overexpressing PI4Kβ, compared with mock-transfected cells. When the same type of experiment was repeated in cells transfected with the catalytically inactive version of PI4Kβ, PI4Kβ(D656A), the response was strongly inhibited (17 ± 7 fF in PI4Kβ(D656A) cells vs. 88 ± 10 fF in mock-transfected cells, n = 5; Fig. 3E). Cotransfection of NCS-1 with wild-type PI4Kβ or PI4Kβ(D656A) did not affect the amplitude of the secretory responses, compared with those obtained in cells expressing wild-type PI4Kβ or PI4Kβ(D656A) alone (Fig. 3E). In analogy with mouse β cells, a robust stimulation of exocytosis elicited by a train of membrane depolarizations was observed in PI4Kβ-overexpressing INS-1E cells (data not shown). To independently confirm our findings by using capacitance measurements, we measured hGH release from INS-1E cells coexpressing hGH and either wild-type PI4Kβ or PI4Kβ(D656A) (Fig. 3F). Overexpression of PI4Kβ increased hGH secretion at 20 mM but not at 3 mM glucose, an effect that was abolished by overexpression of PI4Kβ(D656A). These data indicate that PI4Kβ plays an important role for glucose-dependent priming and, consequently, for the number of granules belonging to the RRP.
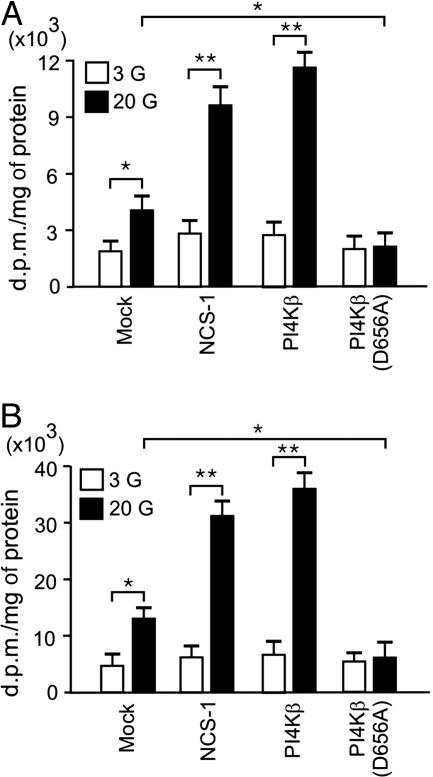
As discussed before, PI4Ks catalyze the first step in the synthesis of PI(4,5)P2, and because PI4Kβ is a downstream target of NCS-1, we have investigated phosphoinositide signaling in INS-1E cells. Under basal conditions, [3H]PI(4)P and [3H]PI(4,5)P2 represented 2% and 4% of the total phosphoinositide pool, respectively. Increasing the glucose concentration from 3 to 20 mM doubled levels of PI(4)P and PI(4,5)P2 (Fig. 4). Interestingly, in the presence of high glucose, overexpression of NCS-1 or PI4Kβ was associated with robust increases in both PI(4)P and PI(4,5)P2 levels (Fig. 4). Consistent with an important role for PI4Kβ in the regulation of phosphoinositide production, expression of the nonfunctional mutant PI4Kβ(D656A) abolished PI(4)P and PI(4,5)P2 synthesis in response to glucose stimulation (Fig. 4). These data are in agreement with a recent report indicating that endogenous PI(4)P and PI(4,5)P2 levels are increased in a stable NCS-1-expressing cell line, compared with control (31). We conclude that glucose stimulates, through activation of NCS-1 and PI4Kβ, the production of PI(4)P and PI(4,5P)2 in β cells and that formed phosphoinositides facilitate the priming of secretory granules.
Fig. 4.
Glucose, NCS-1, and PI4Kβ stimulate PI(4)P and PI(4,5)P2 synthesis in β cells. Intracellular concentrations of [3H]PI(4)P (A) and [3H]PI(4,5)P2 (B) in INS-1E cells transfected with EGFP (mock), NCS-1, PI4Kβ, or PI4Kβ(D656A) in the presence of 3 mM (□) or 20 mM (▪) glucose. Values shown are dpm/mg of protein. Data are presented as mean ± SEM of four experiments (each in triplicate). *, P < 0.05; **, P < 0.01.
The protein CAPS, which is required for Ca2+-dependent exocytosis in β cells (20), has been shown to bind PI(4,5)P2 (21) and may therefore mediate the effects of the lipid on exocytosis. CAPS is expressed in NMRI mouse islets. Lower expression of CAPS was also detected in INS-1E cells (Fig. 5A). Inclusion of an antibody against CAPS (1.5 mg/ml) in the pipette-filling solution strongly inhibited exocytosis in mouse β cells elicited by a train of depolarizations applied 2 min after establishment of the whole-cell configuration (Fig. 5D and ref. 20) but did not influence the magnitude of the whole-cell Ca2+ current (Fig. 5C). Only 2 min was required to obtain full inhibition of the exocytotic response with the applied high concentration of CAPS antibody. Longer infusion times (up to 14 min) were required to obtain full inhibition of the exocytotic response with lower antibody concentrations (0.5 and 1.0 mg/ml; data not shown). No effect was observed with preimmune serum (20). The inhibitory action of the CAPS antibody could not be overcome in cells overexpressing NCS-1 (Fig. 5B) or PI4Kβ (Fig. 5D). These data support the view that NCS-1 and PI4Kβ contribute to the priming of secretory granules by stimulating PI(4,5)P2 synthesis and the recruitment of CAPS to the active zones of release. The exact mechanism by which PI(4,5)P2 and CAPS promote exocytosis remains to be elucidated.
Glucose stimulates insulin secretion in pancreatic β cells by generating changes in metabolic and Ca2+ signaling (17). Our study shed light on the mechanisms by which these signaling pathways converge to tightly control insulin secretion in response to changes in ambient glucose concentration. At high glucose levels, both elevated Ca2+, through NCS-1-mediated PI4Kβ activation and increased ATP/ADP ratio, primarily through ADP-dependent regulation of PI4Kβ, raise PI4Kβ activity, leading to increased production of PI(4)P and, eventually, PI(4,5P)2 through PI4P 5-kinase-catalyzed phosphorylation (Fig. 5E). Formed phosphoinositides promote insulin exocytosis. Thus, PI4Kβ has a unique position in the chain of events that constitute the stimulus-secretion coupling in the pancreatic β cell by integrating signals from two pathways central for the regulation of insulin secretion.
Acknowledgments
We thank Prof. T. F. J. Martin (University of Wisconsin, Madison) for providing us with CAPS antiserum and Prof. C. B. Wollheim (University Medical Center, Geneva, Switzerland) for the INS-1E cells. This work was supported by the Karolinska Institutet, (C.B., B.M., and P.-O.B.), the Family Stefan Persson Foundation (P.-O.B.), the Erik von Kantzow Foundation (P.-O.B.), National Institutes of Health Grant DK-58508 (to P.-O.B.), the Swedish Research Council (B.M. and P.-O.B.), the Swedish Diabetes Association (P.-O.B.), the Novo Nordisk Foundation (C.B.), the Sigurd and Elsa Goljes Foundation (C.B.), the Ragnhild and Einar Lundströms Foundation (C.B.), the Juvenile Diabetes Research Foundation (C.B.), and Aarhus University (J.G.).
Author contributions: J.G., C.B., B.M., and P.-O.B. designed research; J.G., C.B., K.S., A.M.E., J.J., S.A.M., D.-L.W., W.Z., and B.M. performed research; A.J. contributed new reagents/analytic tools; J.G., C.B., A.M.E., J.J., B.M., A.J., and P.-O.B. analyzed data; and J.G., C.B., A.M.E., J.J., A.J., and P.-O.B. wrote the paper.
Abbreviations: CAPS, Ca2+-dependent activator protein for secretion; hGH, human growth hormone; NCS-1, neuronal calcium sensor-1; PI, phosphatidylinositol; PI(4)P, PI 4-phosphate; PI4K, PI 4-kinase; PI(4,5)P2, PI 4,5-bisphosphate; RRP, readily releasable pool.
References
- 1.Chen, X.-L., Zhong, Z.-G., Yokoyama, S., Bark, C., Meister, B., Berggren, P.-O., Roder, J., Higashida, H. & Jeromin, A. (2001) J. Physiol. (London) 532, 649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McFerran, B. W., Graham, M. E. & Burgoyne, R. D. (1998) J. Biol. Chem. 273, 22768-22772. [DOI] [PubMed] [Google Scholar]
- 3.Pongs, O., Lindemeier, J., Zhu, X. R., Theil, T., Endelkamp, D., Krag-Jentgens, I., Lambrecht, H.-G., Koch, K. W., Schwemer, J., Rivosecchi., R., et al. (1993) Neuron 11, 15-28. [DOI] [PubMed] [Google Scholar]
- 4.Rivosecchi, R., Pongs, O., Theil, T. & Mallart, A. (1994) J. Physiol. (London) 474, 223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sippy, T., Cruz-Martin, A., Jeromin, A. & Schweizer, F. E. (2003) Nat. Neurosci. 6, 1031-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendricks, K. B., Wang, B. Q., Schnieders, E. A. & Thorner, J. (1999) Nat. Cell Biol. 1, 234-241. [DOI] [PubMed] [Google Scholar]
- 7.Rajebhosale, M., Greenwood, S., Vidugiriene, J., Jeromin, A. & Hilfiker, S. (2003) J. Biol. Chem. 278, 6075-6084. [DOI] [PubMed] [Google Scholar]
- 8.Taverna, E., Francolini, M., Jeromin, A., Hilfiker, S., Roder, J. & Rosa, P. (2002) J. Cell Sci. 115, 3909-3922. [DOI] [PubMed] [Google Scholar]
- 9.Zhao, X., Varnai, P., Tuymetova, G., Balla, A., Toth, Z. E., Oker-Blom, C., Roder, J., Jeromin, A. & Balla, T. (2001) J. Biol. Chem. 276, 40183-40189. [DOI] [PubMed] [Google Scholar]
- 10.Milosevic, I., Sørensen, J. B., Lang, T., Krauss, M., Nagy, G., Haucke, V., Jahrn, R. & Neher, E. (2005) J. Neurosci. 25, 2557-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holz, R. W., Hlubek, M. D., Sorensen, S. D., Fisher, S. K., Balla, T., Ozaki, S., Prestwich, G. D., Stuenkel, E. L. & Bittner, M. A. (2000) J. Biol. Chem. 275, 17878-17885. [DOI] [PubMed] [Google Scholar]
- 12.Aikawa, Y. & Martin, T. F. J. (2003) J. Cell Biol. 162, 647-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hay, J. C. & Martin, T. F. J. (1993) Nature 366, 572-575. [DOI] [PubMed] [Google Scholar]
- 14.Hay, J. C., Fisette, P. L., Jenkins, G. H., Fukami, K., Takenawa, T., Anderson, R. A. & Martin, T. F. J. (1995) Nature 374, 173-177. [DOI] [PubMed] [Google Scholar]
- 15.Gua, J., Wenk, M. R., Pellegrini, L., Onofri, F., Benfenati, F. & Camilli, P. (2003) Proc. Natl. Acad. Sci. USA 100, 3995-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiedemann, C., Schäfer, T. & Burger, M. M. (1996) EMBO J. 15, 2094-2101. [PMC free article] [PubMed] [Google Scholar]
- 17.Henquin, J. C. (2000) Diabetes 49, 1751-1760. [DOI] [PubMed] [Google Scholar]
- 18.Olofsson, C. S., Gopel, S. O., Barg, S., Galvanovskis, J., Ma, X., Salehi, A., Rorsman, P. & Eliasson, L. (2002) Pflügers Arch. 444, 43-51. [DOI] [PubMed] [Google Scholar]
- 19.Rorsman, P. & Renström, E. (2003) Diabetologia 46, 1029-1045. [DOI] [PubMed] [Google Scholar]
- 20.Olsen, H. L., Høy, M., Zhang, W., Bertorello, A. M., Bokvist, K., Capito, K., Efanov, A. M., Meister, B., Thams, P., Yang, S. N., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 5187-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loyet, K. M., Kowalchyk, J. A., Chaudhary, A., Chen, J., Prestwich, G. D. & Martin, T. F. J. (1998) J. Biol. Chem. 273, 8337-8343. [DOI] [PubMed] [Google Scholar]
- 22.Rupnik, M., Kreft, M., Sikdar, S. K. Grilc, S., Romih, R., Zupancic, Martin, T. F. J. & Zorec, R. (2000) Proc. Natl. Acad. Sci. USA 97, 5627-5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walent, J. H., Porter, B. W. & Martin, T. F. J. (1992) Cell 70, 765-775. [DOI] [PubMed] [Google Scholar]
- 24.Høy, M., Efanov, A. M., Bertorello, A. M., Zaitsev, S. V., Olsen, H. L., Bokvist, K., Leibiger, B., Leibiger, I. B., Zwiller, J., Berggren, P.-O. & Gromada, J. (2002) Proc. Natl. Acad. Sci. USA 99, 6773-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merglen, A., Theander, S., Rubi, B., Chaffard, G., Wollheim, C. B. & Maechler, P. (2004) Endocrinology 145, 667-678. [DOI] [PubMed] [Google Scholar]
- 26.Godi, A., Pertile, P., Meyers, R., Marra, P., Di Tullio, G., Iurisci, C., Luini, A., Corda, D. & De Matteis, M. A. (1999) Nat. Cell Biol. 1, 280-287. [DOI] [PubMed] [Google Scholar]
- 27.Meyers, R. & Cantley L. C. (1997) J. Biol. Chem. 272, 4384-4390. [DOI] [PubMed] [Google Scholar]
- 28.Chilvers, E. R., Batty, I. H., Challiss, R. A. J., Barnes, P. J. & Nahorski, S. R. (1991) Biochem. J. 275, 373-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lilja, L., Johansson, J. U., Gromada, J., Mandic, S. A., Fried, G., Berggren, P.-O. & Bark, C. (2004) J. Biol. Chem. 279, 29534-29541. [DOI] [PubMed] [Google Scholar]
- 30.Jacobsson, G., Bean, A. J., Scheller, R. H., Juntti-Berggren, L., Deeney, J. T., Berggren, P.-O. & Meister, B. (1994) Proc. Natl. Acad. Sci. USA 91, 12487-12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koizumi, S., Rosa, P., Willars, G. B., Challies, R. A. J., Taverna, E., Francolini, M., Bootman, M. D., Lipp, P., Inoue, K., Roder, J., et al. (2002) J. Biol. Chem. 277, 30315-30324. [DOI] [PubMed] [Google Scholar]
- 32.Scalettar, B. A., Rosa, P., Taverna, E., Francolini, M., Tsuboi, T., Terakawa, S., Koizumi, S., Roder, J. & Jeromin, A. (2002) J. Cell Sci. 115, 2399-2412. [DOI] [PubMed] [Google Scholar]
- 33.Tsujimoto, T., Jeromin, A., Saitoh, N., Roder, J. C. & Takahashi, T. (2002) Science 295, 2276-2279. [DOI] [PubMed] [Google Scholar]
- 34.Weiss, O. A., Gibson, G. A., Leung, S. M., Roder, J. & Jeromin, A. (2000) J. Biol. Chem. 275, 24341-24347. [DOI] [PubMed] [Google Scholar]
- 35.Eliasson, L., Renstrom, E., Ding, W. G., Proks, P. & Rorsman, P. (1997) J. Physiol. (London) 503, 399-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O′Callaghan, D. W., Ivings, L., Weiss, J. L., Ashby, M. C., Tepikin, A. V. & Burgoyne, R. D. (2002) J. Biol. Chem. 277, 14227-14237. [DOI] [PubMed] [Google Scholar]