Endocytosis is used by eukaryotic cells to perform a wide range of functions, including the uptake of extracellular nutrients and the regulation of cell-surface receptors, as well as by toxins, viruses, and microorganisms to gain entry into cells (1). Endocytosis actually encompasses many different processes, such as phagocytosis of large (>250 nm) particles as well as pinocytosis of large volumes of fluid (2). One of the most important endocytic mechanisms is a receptor-mediated process whereby the plasma membrane binds specific macromolecules and smaller particles by means of specialized receptors, invaginates around those particles, and then pinches off to form small vesicles. Receptor-mediated endocytosis had been thought to be assisted by specific proteins, either clathrin or caveolin, polymerizing into a spherical shell around the invagination (3). Recently, however, evidence has arisen for a different, clathrin- and caveolin-independent route by which endocytosis may occur (4, 5). The understanding and quantitative analysis of the mechanisms underlying receptor-mediated endocytosis have important implications for not only viral pathogenesis but also the delivery of macromolecules and nanoparticles for intracellular imaging and targeted therapies (6).
A Model for Clathrin-Independent Endocytosis
The key process of endocytosis is the formation of the vesicle wrapping the particle, which requires mechanical force. Despite the essential role of endocytosis in biology, much of the mechanics behind it remains elusive. Although clathrin alone can, under certain conditions, assemble into a caged structure, it may not be the major driving force for membrane deformations during endocytosis. The macromolecular assembly with which clathrin associates, however, does contain proteins that can deform plasma membranes to the degree required (7). Clathrin-independent mechanisms are still rather poorly understood. The study in a recent issue of PNAS by Gao, Shi, and Freund (8) sought to predict the particle size range and kinetics of clathrin-independent endocytosis in a rather general and elegant way, advancing the quantitative understanding of endocytosis, viral budding, and possibly other vesicle-associated biological processes.
In their study, Gao et al. (8) present a mechanical model of endocytosis by considering a particle displaying immobilized ligands gradually attracting and binding receptor proteins on a plasma membrane. The initial binding event nucleates a patch of bound receptors, which holds the particle to the membrane. Unbound (free) receptors on the plasma membrane diffuse toward the edge of the patch and bind particle ligands there, bringing more of the membrane into contact with the particle until the entire particle is engulfed by the plasma membrane (Fig. 1). This process serves as a simple model for the more complicated reactions that occur during endocytosis while retaining much of the interesting dynamics. It is what happens at the boundary of that invagination that dictates these dynamics. Gao et al. assume that all of the free-energy dissipation arises from receptor diffusion, which means that the binding of receptors onto the engulfed particle entails no free-energy change; this assumption is equivalent to saying that the bound and free receptors are in equilibrium at the boundary of the contact zone. With these assumptions and other minor ones, Gao et al. were able to predict the size range of particles that could internalize by means of an endocytic pathway and the associated kinetics (summarized in table 1 of ref. 8).
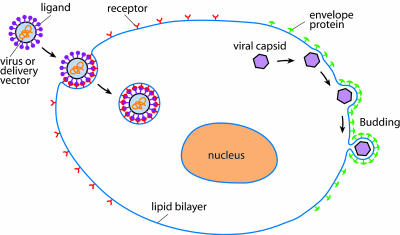
Fig. 1.
A schematic illustration of the receptor-mediated endocytosis and viral budding processes. In modeling clathrin-independent, receptor-mediated endocytosis, Gao et al. (8) assume that once binding between a particle and the plasma membrane is initiated, the particle with immobilized ligands attracts and binds to progressively more receptors on the cell surface. Depletion of free receptors in the vicinity of the contact zone drives diffusion of receptors toward the zone, where they bind particle ligands, bringing more of the membrane into contact with the particle until the entire particle is engulfed by the plasma membrane. With some modifications, this model may be applicable to other biological problems, such as viral budding, in which the viral capsid is wrapped outward into a vesicle by means of membrane-bound envelope proteins.
Salient Features of the Model and the Scaling Laws
The salient features of the model presented by Gao et al. (8) can be understood by simply considering equilibrium between bound and free receptors at the boundary of the contact zone, which is fulfilled whenever receptor diffusion is rate-limiting. The concentration of unbound receptors ξ+ just outside the contact zone is then
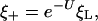 |
[1] |
with ξL the ligand concentration and U the free energy change, in multiples of kBT, that is gained from the binding event and concentrates receptors in the contact zone. U here is the chemical energy eRL released by receptor binding corrected by the elastic bending energy 1/2(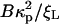 ) (9): i.e.,
) (9): i.e.,
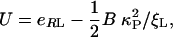 |
[2] |
where B is the bending rigidity of the membrane, and κp is the local curvature. Clearly, we must have ξ+ < ξ0, with ξ0 the far-field concentration of unbound receptors, to drive those receptors toward the bound patch, so a good estimate of minimum radius Rmin for wrapping is given by setting ξ+ = ξ0 in Eq. 1 [which leads to U = -ln(ξ0/ξL)] and, using Eq. 2, resulting in
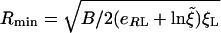 |
[3] |
for cylindrical particles and
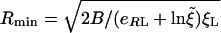 |
[4] |
for spherical particles, with 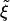 = ξ0/ξL. For ξ = 0.01-0.1 and eRL = 15, as assumed in ref. 8,
= ξ0/ξL. For ξ = 0.01-0.1 and eRL = 15, as assumed in ref. 8, 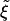 is between -2.3 and -4.6 against 15, so its effect on Rmin is rather small. Note that Rmin given in Eq. 3 is very similar to that in Gao et al. (equation 24 of ref. 8).
is between -2.3 and -4.6 against 15, so its effect on Rmin is rather small. Note that Rmin given in Eq. 3 is very similar to that in Gao et al. (equation 24 of ref. 8).
As R increases from Rmin, receptors right outside the contact zone will be depleted, increasing the concentration differential, which drives receptor diffusion toward the contact interface. In other words, a larger R results in a smaller ξ+, which speeds up receptor diffusion and thus particle wrapping. Once the change in U rises far beyond order unity, however, ξ+ becomes essentially zero, and increasing R only increases the number of receptors that have to diffuse to the particle to envelop it. Thus, we expect the optimal wrapping radius R* to yield an energy change U·kBT of a few kBT. Near R = Rmin, a one kBT increment in free energy results in an ≈3% change in R. Indeed, the values of R* in table 1 of ref. 8 are ≈12-25% higher than the respective Rmin, implying 4-8 kBT of energy driving receptors to bind ligand. We note that a 10-fold change in the unbound receptor density ξ0 gives rise to a 2.3-kBT energy difference, which represents only a minor perturbation to
The model has the potential to shed light on the dynamics of vesicle formation and budding.
Rmin, so minimum and optimal particle sizes are rather insensitive to the value of ξ0.
Because all of the receptors bound to ligands must come from the vicinity of the particle, the diffusion of those receptors provides a good estimate for the time t* required for fully enveloping and ingesting the particle at hand. In this process, receptors are recruited from an area that is roughly the contact area divided by the receptor density ratio 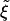 . In the case of cylindrical particles, the characteristic length scale l for the receptor depletion zone is given by l ∼ πR/
. In the case of cylindrical particles, the characteristic length scale l for the receptor depletion zone is given by l ∼ πR/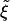 . In the case of spherical particles, we have l ∼ 2R/
. In the case of spherical particles, we have l ∼ 2R/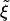 . This length scale, reached by diffusion of receptors, defines a wrapping time t* ∼ l2/D, with D the diffusivity of receptors. These scaling arguments give estimates quite comparable to results in ref. 8.
. This length scale, reached by diffusion of receptors, defines a wrapping time t* ∼ l2/D, with D the diffusivity of receptors. These scaling arguments give estimates quite comparable to results in ref. 8.
Toward a General Quantitative Model for Endocytosis
The more detailed consideration of energy balance, together with an elegant mathematical formulation, allowed Gao et al. (8) to predict the optimal particle size for endocytosis in a minimal cell-based system. Although their model is applicable to a wide range of particle internalization problems, including viral entry and the cellular delivery of nanoparticle probes, it can be further improved by considering more physiologically relevant conditions. For example, effects of osmotic pressure and differential surface tension observed in cellular experiments can be modeled by using a simple per-receptor energy correction (10). More importantly, the “particle” being ingested can be defined rather broadly: all that is required is the growth of a macromolecular complex, such as a clathrin cage, by means of accretion of laterally diffusible components. We believe that the model is equally valid for invaginations outward, as during budding of animal viruses in the last stage of their replication (Fig. 1). As Gao et al. indicate, the model can be modified to include the effect of clathrin and other envelope proteins. In fact, clathrin diffusion to the contact zone is from the cytosol and thus is much faster than the intramembrane diffusion of receptors, so by modifying the free-energy change U in Eq. 2, the above analysis may still be valid.
The model developed by Gao et al. (8) has the potential to shed significant light on the dynamics of vesicle formation and budding involved in very different biological processes, including endocytosis, viral budding, and possibly intracellular sorting and trafficking of vesicles. It is also likely that quantitative models such as that developed in ref. 8 can provide significant insight into the delivery of molecular probes and therapeutic agents into live cells.
Acknowledgments
This work was supported by National Institutes of Health Grant U01 HL080711-01 (to G.B.) and the Fannie and John Hertz Foundation (X.R.B.).
See companion article on page 9469 in issue 27 of volume 102.
References
- 1.Mukherjee, S., Ghosh, R. N. & Maxfield, F. R. (1997) Physiol. Rev. 77, 759-803. [DOI] [PubMed] [Google Scholar]
- 2.Alberts, B., Bray, D., Lewis, J., Raff, M., Roberts, K. & Watson, J. D. (2002) Molecular Biology of the Cell (Garland, New York), 4th Ed.
- 3.Kirchhausen, T. (2002) Cell 109, 413-416. [DOI] [PubMed] [Google Scholar]
- 4.Nichols, B. J. & Lippincott-Schwartz, J. (2001) Trends Cell Biol. 11, 406-412. [DOI] [PubMed] [Google Scholar]
- 5.Lakadamyali, M., Rust, M. J. & Zhuang, X. (2004) Microbes Infect. 6, 929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vyas, S. P., Singh, A. & Sihorkar, V. (2001) Crit. Rev. Ther. Drug Carrier Syst. 18, 1-76. [PubMed] [Google Scholar]
- 7.Nossal, R. & Zimmerberg, J. (2002) Curr. Biol. 12, R770-R772. [DOI] [PubMed] [Google Scholar]
- 8.Gao, H., Shi, W. & Freund, L. B. (2005) Proc. Natl. Acad. Sci. USA 102, 9469-9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shenoy, V. B. & Freund, L. B. (2005) Proc. Natl. Acad. Sci. USA 102, 3213-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rauch, C. & Farge, E. (2000) Biophys. J. 78, 3036-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]