Abstract
Myasthenia gravis is a T cell-dependent, antibody-mediated autoimmune disease. A dual altered peptide ligand (APL) that is composed of the tandemly arranged two single amino acid analogs of two myasthenogenic peptides, p195–212 and p259–271, was demonstrated to down-regulate in vitro and in vivo myasthenia gravis-associated autoreactive responses. The aims of this study were to demonstrate the suppressive properties and to elucidate the mechanism of action of the dual APL on a T cell line specific to the myasthenogenic peptide p195–212. We demonstrate here that incubation of cells of the line with the dual APL resulted in the inhibition of proliferation and secretion of IL-2 and IFN-γ triggered by p195–212. In contrast, secretion of TGF-β and IL-10 was upregulated. The dual APL induced the generation of CD4+CD25+ cells that were characterized by the expression of CD45Rblow, cytotoxic T lymphocyte-associated antigen-4, TGF-β, CD62L, Foxp3, and neuropilin. In addition, the dual APL-treated cells were capable of inhibiting the proliferation response of the line when the two sets of cells were cocultured. The role of CD4+CD25+ cells was further confirmed by demonstrating that the suppression was abrogated by blocking/neutralization of CD25. Thus, the dual APL acts by inducing the formation of CD4+CD25+ regulatory cells. By using a T cell line, we could show that the immunosuppressive CD4+CD25+ cells were indeed induced by the dual APL and are not part of the naturally occurring regulatory cells.
Keywords: immunosuppression, myasthenia gravis, regulatory T cells
Myasthenia gravis (MG) is a B cell-mediated, T cell-dependent autoimmune disease of the neuromuscular junction in which the nicotinic acetylcholine receptor (AChR) is the autoantigen. MG and experimental autoimmune MG (EAMG) are characterized by weakness and fatigability of voluntary muscles (1, 2). The α-subunit of the AChR is predominant for T cell epitopes (3). Thus, peptides p195–212 and p259–271, representing sequences of the human AChR α-subunit, were able to stimulate peripheral blood lymphocytes of patients with MG and were determined to be immunodominant T cell epitopes of SJL and BALB/c mice, respectively (4, 5). A dual altered peptide ligand (APL) composed of the tandemly arranged two single amino acid analogs of the above two peptides was synthesized and was shown to inhibit in vitro and in vivo MG-associated responses (6–11). Furthermore, the dual APL could reverse myasthenogenic manifestations in mice with experimental autoimmune MG induced by pathogenic T cell lines or by the multideterminant molecule Torpedo AChR (8, 12). The down-regulating effects of the dual APL were associated with cytokine immunomodulation, the most significant effect being the up-regulation of TGF-β and down-regulation of IFN-γ secretion (13, 14). The dual APL was also shown to have inhibitory effects on T cell adhesion and on the secretion of matrix metalloproteinase-9 and phospholipase C-γ1 (14, 15). The suppression activity of the dual APL could be actively transferred by splenocytes of dual APL-treated mice (13). Furthermore, the CD4+CD25+ population of regulatory cells was found to be functionally involved in the suppressive action of the dual APL. Administration of the dual APL increased the size of this T cell population in lymph node (LN) of SJL mice. Furthermore, depletion of the CD4+CD25+ T cells diminished significantly the inhibitory effect observed after treatment with the dual APL (16). The dual APL-induced CD4+CD25+ cells were characterized by an increased expression of cytotoxic T-lymphocyte antigen-4 (CTLA-4), intracellular and membranal TGF-β, and Foxp3. Furthermore, treatment with the dual APL resulted in an up-regulation in phosphorylated c-Jun N-terminal kinase. The latter was associated with elevations in Fas- and Fas-L-expressing cells and in the expression of the caspase 8 gene, ultimately leading to apoptosis (17).
CD4+ T cells constitutively expressing the IL-2 receptor α-chain CD25 play a central and prominent role in the maintenance of peripheral tolerance (18). The regulatory CD4+CD25+ T cells play an important role in the maintenance of immune homeostasis and self-tolerance by counteracting the development and effector functions of potentially autoreactive T cells (18). Hence, depletion of this regulatory subset induces the spontaneous onset of various autoimmune disorders (19). Several important molecules, such as glucocorticoid-induced tumor necrosis factor receptor, CTLA-4, TGF-β, CD62L, CD45Rblow, Foxp3, and neuropilin, have emerged as markers of this unique T cell lineage (20–26). These cells act by suppressing the cytokine production and proliferation of conventional CD4+CD25- T cells as well as that of CD8+ T cells. In vitro, these cells are naturally anergic, and they inhibit the proliferation of cocultured conventional CD4+CD25- T cells in a contact-dependent manner (18).
For a better understanding of the role of the dual APL-induced CD4+CD25+ in the mechanism by which it down-regulates MG-associated autoimmune responses, we have established a T cell line specific for the myasthenogenic peptide p195–212. By using the homogenous and specific T cell line, we show in the present study the induction of immunosuppressive CD4+CD25+ cells by the dual APL. The induced CD4+CD25+ cells have the characteristic markers of T regulatory cells, namely, CTLA-4, TGF-β, CD45Rblow, CD62L, Foxp3, and neuropilin. More importantly, the regulatory cells induced by the dual APL are capable of completely suppressing the proliferation response of the myasthenogenic peptide-specific cells of the line when the two sets of cells were cocultured. The demonstration that neutralization/blocking of CD25 abrogates the inhibitory effect confirmed that, indeed, the suppressive effect is due to the CD25+ regulatory population.
Materials and Methods
Mice and Synthetic Peptides. Female mice of the inbred strain SJL (The Jackson Laboratory) were used at the age of 8–12 weeks. The study was approved by the Animal Care and Use Committee of The Weizmann Institute of Science. Peptide p195–212 (DTPYLDITYHFVMQRLPL) was synthesized and characterized as described in ref. 8. The dual APL Lys-262–Ala-207 (VIVKLIPSTSSAVDTPYLDITYHFVAQRLPL) was synthesized (97% purity) by UCB-Bioproducts (Brussels, Belgium). A peptide synthesized in the reversed order (reversed peptide) of the dual APL (LPLRQAVFHYTIDLYPTDVASSTSPILKVIV) was used as control (8).
Establishment of a p195–212-Specific T Cell Line. LN cells were harvested from SJL mice 10 days after their immunization with p195–212 [10 μg per mouse in complete Freund's adjuvant (Difco)]. The LN cells (n = 5 × 107) were cultured in 25-ml flasks in 5 ml of enriched RPMI medium 1640 supplemented with 1% normal mouse serum and 5 μg/ml p195–212. After 4 days of incubation, cells were washed and resuspended in 5 ml of enriched RPMI medium 1640 supplemented with 10% FCS and 2 ng/ml of recombinant murine IL-2 (PeproTech, Rocky Hill, NJ). Cells were exposed to the stimulating peptide (5 μg/ml) presented on irradiated (3,000 rad) syngeneic spleen cells every 14 days (27) and were maintained between stimulations in enriched medium containing IL-2. For all of the experiments, cells of the line were harvested 7 days after antigenic stimulation.
Proliferative Responses of the T Cell Line and Stimulation and Inhibition of Cytokines. Cells (n = 104 per well) of the line were cultured with 0.5 × 106 irradiated (3,000 rad) syngeneic spleen cells in the presence of various concentrations of p195–212. For inhibition of proliferative response, cells were stimulated with a single dose of antigen (1 μg/ml), and various concentrations of the dual or reversed dual APL were added. In some cases, cells of the line were cocultured with various numbers of dual or reversed dual APL preincubated (24 h) cells; in certain experiments, cells were preincubated with dual APL in the presence of the neutralizing/blocking anti-CD25 antibody or its matched isotype control at a concentration of 50 μg/ml for 24 h. Cultures were set in 200 μl of enriched RPMI medium 1640 medium containing 10% FCS and incubated for 48 h, after which 0.5 μCi (1 Ci = 37 GBq) of [3H]thymidine (5 Ci/mmol; Amersham Biosciences) was added. Cells were harvested, and radioactivity was counted 16 h later. For cytokine production, cells of the line (n = 104 cells per ml) were cultured in enriched RPMI medium 1640 containing 10% FCS, with irradiated (3,000 rad) syngeneic mouse splenocytes in the presence of 1 μg/ml p195–212 with or without the dual APL or the reversed dual APL (500 μg/ml) and incubated for 24–48 h. Thereafter, supernatants were collected and analyzed for cytokine content by ELISA using the relevant standard capture and detecting antibodies (OptEIA, Pharmingen and R & D Systems).
Antibodies and Fluorescence Staining of T Cells. The following antibodies were used in the study: anti-CD4-phycoerythrin (PE) (GK1.5), anti-CD25/IL-2 receptor-FITC (7D4), anti-CD28-FITC (37.51), anti-CD152/CTLA-4-PE (1B8), and their matched isotype controls (Southern Biotechnology Associates); anti-CD45Rb-PE (16A), anti-CD62L-PE (MEL14), anti-CD25 (PC61), and their matched isotype controls (Pharmingen); anti-TGF-β-PE (TB21) and its matched isotype control (IQ Products, Groningen, The Netherlands); and anti-neuropilin antibody (H-286) and its matched isotype control (Santa Cruz Biotechnology). Cells (n = 5 × 105) were washed with 10% FCS in PBS, incubated with the relevant antibody, washed again, and analyzed by FACS (Becton Dickinson). For intracellular staining, cells were first incubated with a fixation solution, washed, and resuspended in permeabilization solution (Serotec) in the presence of their respective antibodies.
Preparation of Cell Lysates and Western Blot Analysis. Cells (n = 5 × 106) were incubated in 50 μl of lysis buffer, pH 7.2 (50 mM Hepes/150 mM NaCl/1.5 mM MgCl2/1 mM EGTA/1% EDTA/1% Triton X-100/10% glycerol/1 mM Na-orthovanadate/30 mM Na-pyrophosphate/1 mM PMSF/10 μg/ml leupeptin/10 μg/ml aprotinin) for 10 min on ice. Supernatants were collected, and protein concentrations were determined by using Bradford reagent (Bio-Rad). Lysates were boiled in sample buffer, and equal amounts of proteins were separated on 10% SDS-polyacrylamide gel and transferred to nitrocellulose membrane. After blocking, the membrane was reacted with the relevant antibody and further with the secondary antibody coupled to horseradish peroxidase. Detection was carried out with enhanced chemiluminescence. Protein expression was determined by photodensitometry with National Institutes of Health image software.
Real-Time PCR. Levels of mRNA of Foxp3 were analyzed by quantitative real-time RT-PCR using LightCycler (Roche, Mannheim, Germany). Reverse transcription into complementary DNA was performed by using Molony murine leukemia virus reverse transcriptase (Promega). Real-time PCR was performed according to the manufacturer's instructions. Briefly, 20 μl of reaction volume contained 3 mM MgCl2, LightCycler HotStart DNA SYBR Green I mix (Roche), specific primer pairs, and 5 μl of cDNA. PCR cycling conditions were 10 min at 95°C followed by 45 cycles of 95°C for 15 sec, 60°C for 15 sec and 72°C for 15 sec. The following primers were used: 5′-taccacaatatgcgaccc-3′ and 5′-ctcaaattcatctacggtcc-3′ (Foxp3) and 5′-gacgttgacatccgtaaag-3′ and 5′-ggccggactcatcgta-3′ (β-actin).
Results
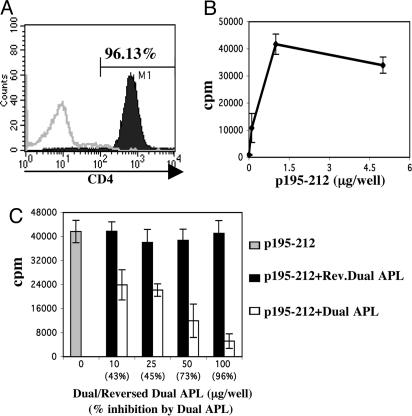
The Dual APL Inhibits Proliferation of the p195–212 Myasthenogenic T Cell Line and Immunomodulates Cytokine Secretion. The established p195–212-specific T cell line was first characterized for its purity and specificity. As is shown in Fig. 1A, the generated T cell line was determined to consist of 96% CD4+ cells. To determine the ability of the line to proliferate to p195–212, we tested its proliferative response to various concentrations of the peptide in the presence of irradiated splenocytes used as antigen-presenting cells. Fig. 1B depicts the dose–response proliferation profile of the line cells to p195–212. Because maximum proliferation was reproducibly observed with 1 μg per well (5 μg/ml), we used this concentration for all experiments of the study. Furthermore, to determine the ability of the dual APL to inhibit the p195–212-specific proliferation of the T cell line, we stimulated cells of the line in the presence or absence of various concentrations of the dual APL or the control reversed dual APL. As can be seen in Fig. 1C, the dual APL inhibited proliferation of the line in a dose-dependent manner, with the maximum inhibition of 96% observed at a concentration of 500 μg/ml. We therefore used this concentration for further experiments to study the effects of the dual APL. It is noteworthy that the reversed dual APL, which served as control peptide, did not inhibit at all or inhibited to an insignificantly lesser extent the proliferative response of the stimulated T cells.
Fig. 1.
Characterization of the p195–212-specific T cell line. (A) SJL mice were immunized with p195–212 (10 μg per mouse in complete Freund's adjuvant), and LN cells were harvested 10 days later. p195–212-specific T cell line was established and maintained as described in Materials and Methods. Cells were stained with PE-coupled anti-CD4 specific antibody 7 days after antigenic stimulation and analyzed by FACS. (B) Cells of the line (n = 104 per well) were stimulated with various concentrations of p195–212 in the presence of irradiated syngeneic splenocytes (n = 0.5 × 106 per well). (C) Cells were incubated with p195–212 (1 μg per well) in the presence or absence of various doses of the dual APL or the reversed dual APL. (B and C) The proliferation assay was performed as described in Materials and Methods. Results are expressed as mean cpm of triplicates ± SD values, and they represent one experiment of four performed.
T helper 1 cytokines IFN-γ and IL-2 have been shown to be involved in the pathogenesis of experimental autoimmune MG (28, 29). To test the effect of the dual APL on the p195–212-specific T cells, cells of the line were incubated with p195–212 with or without the dual or reversed dual APL for 24 (IFN-γ, IL-2, and IL-10) or 48 (TGF-β) h. As shown in Fig. 2, the dual APL inhibited significantly the secretion of IFN-γ and IL-2 in parallel to the up-regulation of TGF-β and IL-10. In contrast, the reversed dual APL had no effect on cytokine secretion.
Fig. 2.
The effect of the dual APL on cytokine secretion by the p195–212-specific T cells. Cells of the line (n = 106 per ml) were stimulated with p195–212 (5 μg per ml) with or without the dual APL or the reversed dual APL (500 μg per ml) for 24–48 h. Supernatants were collected, and concentrations of IFN-γ (A), IL-2 (B), TGF-β (C), and IL-10 (D) were determined by ELISA. Results are mean concentrations of triplicates ± SD values. *, P < 0.005; **, P < 0.0001; as compared with supernatants of cells stimulated with p195–212 alone.
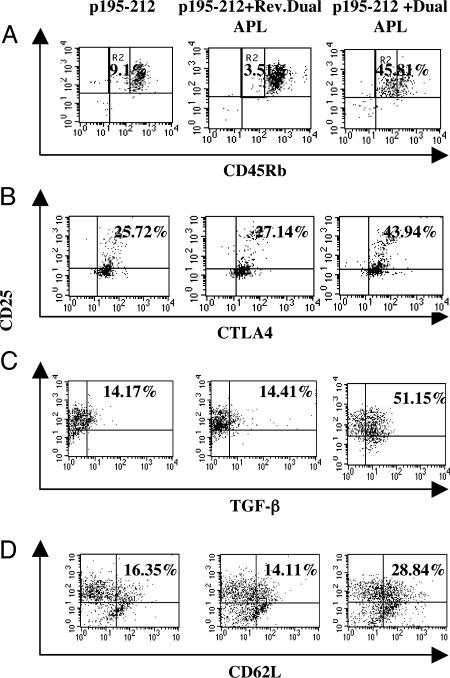
The Dual APL Decreases the Expression of CD28 on the Myasthenogenic Peptide-Specific Cells of the Line and Up-Regulates Cells Expressing Regulatory Markers. CD28 is constitutively expressed on T cells as an activating costimulatory molecule. CD28 expression was determined by using FACS analysis after 24-h stimulation of the line cells with p195–212 in the presence or absence of the dual or reversed dual APL. As can be seen in Fig. 3, addition of the dual APL resulted in a 2-fold down-regulation of CD28 expression on the line cells (p195–212, 36.49%; p195–212 plus dual APL, 17.81%). The reversed dual APL did not have any effect on CD28 expression. Because in vivo experiments indicated that treatment with dual APL up-regulated CD4+CD25+ regulatory cells (16, 17), we tested on the CD25+ population of the line for various markers that have been implicated in the regulatory mechanism of the CD4+CD25+ cells. Fig. 4A is an analysis of the CD45Rbhigh and CD45Rblow isoforms in the untreated, reversed dual APL-treated, and the dual APL-treated groups. It can be seen that the CD45Rbhigh/CD45Rblow population ratio observed for the untreated and control peptide-treated groups is almost completely reversed in the dual APL-treated cells, with the percentages of the CD45Rblow being 9.1% and 3.51% for the untreated and reversed dual APL-treated groups, respectively, in contrast with 45.81% seen in the dual APL-treated cells. Alternatively, the CD45Rbhigh percentages were determined to be 82.59%, 94.51%, and 42.33% for untreated, reverse dual APL-treated, and dual APL-treated groups, respectively. It is worth mentioning that when the cells of the line were incubated with only the dual APL, the CD45Rblow expression was 8.82%, similar to the expression levels of untreated cells of the line, which suggests that the dual APL acts mainly on the p195–212-activated cells.
Fig. 3.
The dual APL down-regulates the expression of CD28. Cells (n = 106 per ml) of the line were stimulated with p195–212 (5 μg per ml) with or without the dual or the reversed dual APL (500 μg per ml). After 24 h, cells were stained with FITC-coupled anti-CD28 antibody and analyzed by FACS. Results are presented as the percentage of CD28 cells, and they represent one of two experiments performed.
Fig. 4.
The dual APL up-regulates regulatory markers on p195–212-specific T cells. Cells (n = 106 per ml) of the line were stimulated with p195–212 (5 μg per ml) with or without the dual or reversed dual APL (500 μg per ml) for 24 h. Cells were double-stained for CD25 and CD45Rb (A), intracellular CTLA-4 (B), intracellular TGF-β (C), or CD62L (D). The results in A are presented as the percentage of CD45Rblow gated as R2. Results represent one of two experiments performed.
Because CD45Rblow is known to up-regulate the expression of the inhibitory costimulatory molecule CTLA-4 and, furthermore, because CTLA-4 has been reported to be expressed on naturally occurring regulatory CD4+CD25+ cells (30), we looked for the effect of the dual APL on CTLA-4 expression. Fig. 4B demonstrates an up-regulation in CTLA-4 expression from 25.7% determined for the p195–212 stimulated, untreated T cell population to 43.9% observed after the addition of the dual APL. Because CTLA-4-mediated T cell suppression is linked to TGF-β and because we showed that TGF-β is upregulated by the dual APL, we wanted to see its status in the CD25+ cells. To this end, cells were stimulated for 24 h with p195–212 in the absence or presence of the dual APL and then intracellularly stained for TGF-β. A marked elevation of 51.15% of TGF-β was observed in the CD25+ regulatory population induced by the dual APL as compared with 14.17% and 14.41%, respectively, in the untreated and the reversed dual APL-treated cells (Fig. 4C). Next, the cells were stained for expression of CD62L reported to be down-regulated from cell surface upon cell activation (24). The dual APL caused a nearly 2-fold up-regulation (Fig. 4D) of CD62L (28.84%) in comparison with untreated cells (16.35%) or cells treated with the reversed dual APL (14.11%).
To further confirm that the up-regulated population is indeed CD4+CD25+, we tested the expression of Foxp3, a transcription repressor protein molecule that is exclusively confined to CD4+CD25+ cells (21, 22). Results shown in Fig. 5A represent mRNA expression levels of Foxp3 normalized to β-actin and presented as the percentage of the levels in the p195–212-stimulated cells (accounted as 100%). The figure demonstrates an elevation of ≈6-fold in Foxp3 expression in the dual APL-treated cells when compared with those treated with reversed dual APL or to cells stimulated with p195–212 alone. Because another gene coding for neuropilin was reported to be coexpressed with Foxp3 and has also been implicated in in vitro suppressor function (26), we determined its expression as well. Thus, cell lysates were subjected to Western blot and analyzed with National Institutes of Health image software. Fig. 5B shows the levels of neuropilin that have been normalized to β-actin. In parallel to Foxp3, neuropilin is also up-regulated in the dual APL-treated population of cells.
Fig. 5.
Foxp3 and neuropilin are up-regulated in p195–212-specific T cells by the dual APL. T cells (n = 106 per ml) of the line were stimulated with p195–212 (5 μg per ml) with or without the dual or the reversed dual APL (500 μg per ml) for 24 h. (A) Total RNA was extracted, and real-time PCR was performed for Foxp3 expression as described in Materials and Methods. Results are expressed as the mean percentage of the gene expression of triplicates ± SD values. *, P < 0.0005 when gene expression in the presence of the dual APL was compared with that in the absence of the dual APL. (B) Whole-cell lysates were prepared, separated on SDS/PAGE, and transferred to a nitrocellulose membrane that was later blotted with anti-neuropilin or anti-β-actin. Results are expressed as densitometry units (measured with National Institutes of Health image software), and they represent one of two experiments performed.
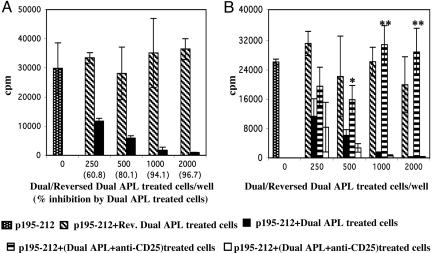
The Dual APL-Induced CD25+ Regulatory Cells Suppress Proliferation of p195–212-Specific T Cell Line. To assess the suppressive capacity of the dual APL-induced CD25+ cells, we cocultured the latter cells with cells of the line that were not preincubated with the dual APL. Fig. 6A demonstrates the dose-dependent manner in which the dual APL-treated cells inhibited the proliferation of p195–212 stimulated cells. A 50% inhibition of the proliferation of the T cell line (104 cells) was achieved with only 500 dual APL-pretreated cells. An almost complete (96%) inhibition was observed when ≥1,000 dual APL-treated cells were cocultured with 10,000 p195–212-stimulated T cells. In contrast, the control reversed dual APL-treated cells did not inhibit proliferation at all or inhibited proliferation to an insignificantly lesser extent. To further confirm that the dual APL-induced immunoregulatory cells are indeed the CD25+ subset of cells, we used anti-mouse CD25 to block/neutralize the IL-2α receptor CD25. To this end, stimulation and treatment of the line cells with the dual APL was carried out in the presence of anti-CD25 or its matched isotype control. Fig. 6B shows that the inhibitory capacity of the dual APL-induced T cells was significantly abrogated upon treatment with anti-CD25. Fig 6 also shows that the control isotype did not affect the inhibitory capacity of the dual APL-treated cells. These results support the notion that the dual APL has conferred suppressive properties to the p195–212-specific T cells by converting them to CD4+CD25+ immunoregulatory cells.
Fig. 6.
Dual APL-treated cells inhibit the proliferation of p195–212-specific T cell line. (A) Cells (n = 104 per well) of the line were cocultured with various numbers of reversed dual APL or dual APL-treated cells (described in Materials and Methods) in the presence of p195–212 (1 μg per well) and antigen-presenting cells. (B) Cells of the line were cocultured with reversed dual APL-treated cells or dual APL (in the absence or presence of anti-CD25 or its matched isotype control) treated cells with p195–212 and antigen-presenting cells. A proliferation assay was performed as described in Materials and Methods. Results are expressed as mean cpm of triplicates ± SD values, and they represent one of four experiments performed. *, P < 0.05; **, P < 0.0001; proliferation with dual APL plus anti-CD25 treated cells was compared with that of dual APL treated cells.
Discussion
The main findings of the present study are that the dual APL induced the generation of CD4+CD25+ cells in a T cell line specific for the myasthenogenic peptide p195–212. The CD4+CD25+ cells expressed elevated levels of characteristic regulatory markers, such as CD45Rblow, CTLA-4, TGF-β, CD62L, Foxp3 gene, and neuropilin. Furthermore, cells of the line that were treated with the dual APL inhibited the proliferation of p195–212-specific T cells when the two sets of cells were cocultured. The demonstration that the suppression was abrogated by anti-CD25 neutralizing/blocking antibodies confirmed that the dual APL-induced cells are indeed immunoregulatory CD4+CD25+ cells.
The dual APL down-regulated the production of the T helper 1-type cytokines (IL-2 and IFN-γ) triggered by the myasthenogenic peptide and up-regulated IL-10 and the immunosuppressive TGF-β. This shift in cytokine pattern caused by the dual APL was earlier observed in in vivo studies as well, which used SJL (13) and BALB/c (14) mice immunized with p195–212 and p259–271, respectively demonstrating that the active suppression exerted by the dual APL is mediated partially by the modulated cytokine secretions.
Incubation of the p195–212-specific T cell line with the dual APL resulted in up-regulated expression of regulatory markers. One of the most pronounced effects of the dual APL was the shift of the CD45high isoform to the low-density cells (CD45Rblow). CD45Rblow has been credited with being a specific marker of the CD4+CD25+ cells, a potent immunomodulatory target, and a causative molecule for the enhancement of intracellular CTLA-4 (30). The specific immunomodulatory properties of CD34Rblow are mediated by IL-10 and TGF-β (31, 32). Although the lower form is implicated in the regulatory mechanism of CD4+CD25+ regulatory cells, CD45Rbhigh is typically present on activated cells (33, 34). Thus, a significant number of the activated cells have been converted to CD45Rblow regulatory cells by the action of the dual APL. Because the dual APL has immensely up-regulated CD45Rblow, which was reported to mediate its suppressive activity by means of IL-10 and TGF-β (31, 32), it is likely that the in vitro suppression by the dual APL is at least partially cytokine-dependent. Most in vitro studies of murine and human CD25+ T cells support a cytokine-independent mechanism of suppression, but in some cases IL-10, IL-4, and TGF-β have been implicated in mediating in vitro suppression (18).
The dual APL down-regulates the expression of CD28 and, thus, reduces the costimulatory signal required for promoting IL-2 formation and, hence, cell expansion (35). CTLA-4, which shares the same ligand (B7) as that of CD28, was shown to be up-regulated by the dual APL. Given its higher affinity for B7, CTLA-4 might out-compete CD28 when B7 expression is limiting, resulting in decreased CD28 signaling (35). CTLA-4 acts as an inhibitory costimulating molecule, which, by cross-linking, enhances production of the inhibitory cytokine TGF-β by activated cells (25). Up-regulation of CTLA-4 has been linked to CD45 through the calcineurin-mediated signaling pathway (30). Fecteau et al. (30) associated their 2-fold increase in the number of CTLA-4-expressing CD4+ cells with an increase in CD45Rblow expression and suggested that CD45Rblow isoforms act through CTLA-4 up-regulation. Read et al. (34) showed in their studies on autoimmune colitis that CTLA-4 is expressed constitutively on CD4+CD25+CD45Rblow cells. Although CTLA-4 is constitutively expressed at a high level on T regulatory cells, it is also highly expressed on recently activated CD4+ cells. Nevertheless, it is rapidly down-regulated on activated CD4+ cells, whereas its expression is stronger and lasts longer on regulatory T cells (35). Thus, in the present study, it is likely that the relatively high levels of CTLA-4 (25.72%) expressed by cells of the line (untreated with the dual APL) are due to recent (24 h) activation with p195–212.
The dual APL caused the up-regulation of yet another recently defined regulatory molecule, CD62L. CD62L is a selectin adhesion molecule whose expression crucially determines the LN homing capacity of cells, including CD4+CD25+ cells, thus enabling them to mediate their suppressive activity in a site-specific manner (36). CD62L+ regulatory T cells were reported to play a unique role in the control of autoimmune diabetes (36, 24). Unlike other leukocyte adhesion proteins, CD62L is rapidly down-regulated from the cell surface upon T cell activation, whereas it is highly expressed on the surface of 50–60% of the CD4+CD25+ cells (24). Although the exact mechanism by which the CD62L+subset of cells exert their suppressive activity is still ill-defined, reports link them to increased production of TGF-β (36).
Foxp3 gene, which is significantly up-regulated (6-fold) by the dual APL, encodes a transcription repressor protein. Foxp3 gene is predominantly restricted to the CD4+CD25+ population in the thymus and periphery, and reports have linked it to the development and function of this T cell subset (21, 22). Furthermore, in contrast with other molecular markers of the CD4+CD25+ cells, such as CTLA-4, CD25, and glucocorticoid-induced tumor necrosis factor receptor, Foxp3 is not up-regulated in activated T cells (22) and is therefore considered the best marker for these cells. More importantly, Foxp3 has been correlated with regulatory activity in many autoimmune diseases, including MG (37), where decreased levels of Foxp3 gene expression in MG patients have been reported. It is worth noting that another set of regulatory cells, namely the Tr1 population, do not express Foxp3 (38), although they have some similar properties to the CD4+CD25+ cells, which supports the notion that the regulatory cell subset induced by the dual APL may indeed be CD4+CD25+.
Neuropilin, a neurological protein that has been recently reported to be coexpressed with foxp3 and significantly down-regulated after T cell activation (26) was also up-regulated by the dual APL. Neuropilin has been identified as a potentially useful and specific marker on CD4+CD25+ (26). However, unlike foxp3, there have been no reports linking neuropilin to regulatory activity in autoimmune diseases.
In many autoimmune and inflammatory conditions, it has been shown that suppression of T cell proliferation is an exclusive property of CD4+CD25+ cells (39). Indeed, the dual APL-induced CD25+ cells completely and efficiently suppressed the proliferation of the myasthenogenic peptide-specific cells of the line. More importantly, the abrogation of suppression demonstrated by blocking/neutralizing the IL-2α receptor CD25 indicate that the regulatory cells induced by the dual APL are enriched within the same phenotypic subset of CD4+CD25+.
It is noteworthy that only the dual APL was capable of inducing the CD4+CD25+ regulatory cells, because the control reversed dual APL did not change the levels of these cells when compared with untreated cells of the line. Furthermore, the dual APL-treated splenocytes were shown to efficiently inhibit the proliferative responses of mice immunized with p195–212. However, the splenocytes did not inhibit the proliferative responses of LN cells of mice immunized with another antigen (E.M., unpublished data), namely, the monoclonal anti-DNA antibody that expresses the 16/6 Id and is capable of inducing systemic lupus erythematosus (40). The latter results suggests that the dual APL-induced CD4+CD25+ cells are specific in their mechanism of action.
Based on the accumulating reported data and on the results presented in this study, it may be proposed that treatment of the myasthenogenic peptide-specific T cells with the dual APL polarizes the T cells toward a CD4+CD25+ regulatory phenotype, characterized by the expression of CD45Rblow, CTLA-4, TGF-β, Foxp3, and neuropilin, to secure immunotolerance. The dual APL acts by initially converting the CD45Rbhigh (on the activated cells) to the lower isoform, which mediates its suppressive activity either via IL-10 or by raising intracellular CTLA-4 expression. CTLA-4 further causes elevation of the immunosuppressive cytokine TGF-β. Most importantly, by using a specific and homologous population of cells, we show that the immunosuppressive CD4+CD25+ cells are indeed induced by the dual APL and are not part of the naturally occurring regulatory cells. MG is a prototypical T cell-dependent autoimmune disease for which a functional defect and a decrease in the number of circulating CD4+CD25+ have been reported (37). The induction of regulatory T cells is therefore a major goal in the treatment of autoimmune conditions, like MG, especially when it is specific, as opposed to the current use of nonspecific drugs. The regulatory population of cells induced by the dual APL is similar in cell surface phenotypes to the naturally occurring CD25+ T regulatory cells. Thus, the ability of the dual APL to suppress myasthenogenic T cell responses through the induction of CD4+CD25+ cells further suggests its significant therapeutic potential.
Acknowledgments
This study was supported by DeveloGen (Rehovot, Israel).
Author contributions: B.V.A. and E.M. designed research; B.V.A. performed research; B.V.A. and E.M. analyzed data; B.V.A. and E.M. wrote the paper; and M.S. participated in discussions.
Abbreviations: APL, altered peptide ligand; CTLA-4, cytotoxic T-lymphocyte antigen-4; LN, lymph node; MG, myasthenia gravis.
References
- 1.Lindstrom, J. (1985) Annu. Rev. Immunol. 3, 109-131. [DOI] [PubMed] [Google Scholar]
- 2.Drachman, D. B. (1994) N. Engl. J. Med. 25, 1797-1810. [DOI] [PubMed] [Google Scholar]
- 3.Lindstrom, J. D., Shelton, D. & Fuji, Y. (1988) Adv. Immunol. 42, 233-284. [DOI] [PubMed] [Google Scholar]
- 4.Brocke, S., Brautbar, C., Steinman, L., Abramsky, O., Rothbard, J., Neumann, D., Fuchs, S. & Mozes, E. (1988) J. Clin. Invest. 82, 1894-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brocke, S., Dayan, M., Rothbard, J., Fuchs, S. & Mozes, E. (1990) Immunology 69, 495-500. [PMC free article] [PubMed] [Google Scholar]
- 6.Katz-Levy, Y., Kirshner, S. L., Sela, M. & Mozes, E. (1993) Proc. Natl. Acad. Sci. USA 90, 7000-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirshner, S. L., Zisman, E., Fridkin, M., Sela, M. & Mozes, E. (1996) Scand. J. Immunol. 44, 512-521. [DOI] [PubMed] [Google Scholar]
- 8.Katz-Levy, Y., Paas-Rozner, M., Kirshner, S., Dayan, M., Zisman, E., Fridkin, M., Wirguin, I., Sela, M. & Mozes, E. (1997) Proc. Natl. Acad. Sci. USA 94, 3200-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz-Levy, Y., Dayan, M., Wirguin, I., Fridkin, M., Sela, M. & Mozes, E. (1998) J. Neuroimmunol. 85, 78-86. [DOI] [PubMed] [Google Scholar]
- 10.Karni, A., Zisman, E., Katz-Levy, Y., Paas-Rozner, M., Dayan, M., Brautbar, C., Abramsky, O., Sela, M. & Mozes, E. (1997) Neurology 48, 1638-1642. [DOI] [PubMed] [Google Scholar]
- 11.Dayan, M., Sthoeger, Z., Neiman, A., Abarbanel, J., Sela, M. & Mozes, E. (2004) Hum. Immunol. 65, 571-577. [DOI] [PubMed] [Google Scholar]
- 12.Paas-Rozner, M., Dayan, M., Paas, Y., Changeux, J. P., Wirguin, I., Sela, M. & Mozes, E. (2000) Proc. Natl. Acad. Sci. USA 97, 2168-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paas-Rozner, M., Sela, M. & Mozes, E. (2001) Proc. Natl. Acad. Sci. USA 98, 12642-12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faber-Elmann, A., Grabovsky, V., Dayan, M., Sela, M., Alon, R. & Mozes, E. (2000) Int. Immunol. 12, 1651-1658. [DOI] [PubMed] [Google Scholar]
- 15.Faber-Elmann, A., Grabovsky, V., Dayan, M., Sela, M., Alon, R. & Mozes, E. (2001) FASEB J. 15, 187-194. [DOI] [PubMed] [Google Scholar]
- 16.Paas-Rozner, M., Sela, M. & Mozes, E. (2003) Proc. Natl. Acad. Sci. USA 100, 6676-6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-David, H., Sela, M. & Mozes, E. (2005) Proc. Natl. Acad. Sci. USA 102, 2028-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shevach, E. M. (2001) J. Exp. Med. 193, 41-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 155, 1151-1164. [PubMed] [Google Scholar]
- 20.Takahashi, T., Tagami, T., Yamazaki, S., Uede, T., Shimizu, J., Sakaguchi, N., Mak, T. W. & Sakaguchi, S. (2000) J. Exp. Med. 192, 303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khattri, R., Cox, T., Yasayko, S. A. & Ramsdell, F. (2003) Nat. Immunol. 4, 337-342. [DOI] [PubMed] [Google Scholar]
- 22.Hori, S., Nomura, T. & Sakaguchi, S. (2003) Science 299, 1057-1061.12522256 [Google Scholar]
- 23.Shimizu, J., Yamazaki, S., Takahashi, T., Ishida, Y. & Sakaguchi, S. (2002) Nat. Immunol. 3, 135-142. [DOI] [PubMed] [Google Scholar]
- 24.Fu, S., Yopp, A. C., Mao, X., Chen, D. M., Zhang, N., Chen, D., Mao, M. W., Ding, Y. Z. & Bromberg, J. S. (2004) Am. J. Transplant. 4, 65-78. [DOI] [PubMed] [Google Scholar]
- 25.Gomes, N. A., Gattass, C. R., Barreto-de-Souza, V., Wilson, M. E. & DosReis, G. A. (2000) J. Immunol. 164, 2001-2008. [DOI] [PubMed] [Google Scholar]
- 26.Bruder, D., Probst-Kepper, M., Westendorf, A. M., Geffers, R., Beissert, S., Loser, K., von Boehmer, H., Buer, J. & Hansen, W. (2004) Eur. J. Immunol. 34, 623-630. [DOI] [PubMed] [Google Scholar]
- 27.Axelrod, O. & Mozes, E. (1986) Immunobiology 172, 99-109. [DOI] [PubMed] [Google Scholar]
- 28.Wang, H. B., Shi, F. D., Li, H., van der Meide, P. H., Ljunggren, H. G. & Link, H. (2000) Clin. Immunol. 95, 156-162. [DOI] [PubMed] [Google Scholar]
- 29.Okumura, S., McIntosh, K. & Drachman, D. B. (1994) Ann. Neurol. 36, 704-713. [DOI] [PubMed] [Google Scholar]
- 30.Fecteau, S., Basadonna, G. P., Freitas, A., Ariyan, C., Sayegh, M. H. & Rothstein, D. M. (2001) Nat. Immunol. 2, 58-63. [DOI] [PubMed] [Google Scholar]
- 31.Smart, V., Foster, P. S., Rothenberg, M. E., Higgins, T. J. V. & Hogan, S. P. (2003) J. Immunol. 171, 2116-2126. [DOI] [PubMed] [Google Scholar]
- 32.Davies, J. D., O'Connor, E., Hall, D., Krahl, T., Trotter, J., Davies, J. D., O'Connor, E., Hall, D., Krahl, T., Trotter, J., et al. (1999) J. Immunol. 163, 5353-5357. [PubMed] [Google Scholar]
- 33.Ariyan, C., Salvalaggio, P., Fecteau, S., Deng, S. Y., Rogozinski, L., Mandelbrot, D., Sharpe, A., Sayegh, M. H., Basadonna, G. P. & Rothstein, D. M. (2003) J. Immunol. 171, 5673-5677. [DOI] [PubMed] [Google Scholar]
- 34.Read, S., Malmstrom, V. & Powrie, F. (2000) J. Exp. Med. 192, 295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothstein, D. M. & Sayegh, M. H. (2003) Immunol. Rev. 196, 85-108. [DOI] [PubMed] [Google Scholar]
- 36.You, S., Slehoffer, G., Barriot, S., Bach, J. F. & Chatenoud, L. (2004) Proc. Natl. Acad. Sci. USA 101, 14580-14585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balandina, A., Lecart, S., Dartevelle, P., Saoudi, A. & Berrih-Aknin, S. (2005) Blood 105, 735-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vieira, P. L., Christensen, J. R., Minaee, S., O'Neill, E. J., Barrat, F. J., Boonstra, A., Barthlott, T, Stockinger, B., Wraith, D. C. & O'Garra, A. (2004) J. Immunol. 172, 5986-5993. [DOI] [PubMed] [Google Scholar]
- 39.Wraith, D. C., Nicolson, K. S. & Whitley, N. T. (2004) Curr. Opin. Immunol. 16, 695-701. [DOI] [PubMed] [Google Scholar]
- 40.Mendlovic, S., Brocke, S., Shoenfeld, Y., Ben-Bassat, M., Meshorer, A., Bakimer, R. & Mozes, E. (1988) Proc. Natl. Acad. Sci. USA 85, 2260-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]