Abstract
Dopamine neurotransmission has been implicated in the modulation of many cognitive processes. Both rapid (phasic) and slower (tonic) changes in its extracellular concentration contribute to its complex actions. Fast in vivo electrochemical techniques can measure extracellular dopamine on a rapid time scale but without the selectivity afforded with slower techniques that use chemical separations. Cyclic voltammetry improves chemical resolution over other electrochemical methods, and it can resolve dopamine changes in the brains of behaving rodents over short epochs (<10 s). With this method, however, selective detection of slower dopamine changes is still elusive. Here we demonstrate that principal component regression of cyclic voltammetry data enables quantification of changes in dopamine and extracellular pH. Using this method, we show that cocaine modifies dopamine release in two ways: dopamine concentration transients increase in frequency and magnitude, whereas a gradual increase in steady-state dopamine concentration occurs over 90 s.
Keywords: cyclic voltammetry, nucleus accumbens, principal component regression
Fast changes in the extracellular concentration of neurotransmitter can arise from phasic neuronal firing, whereas long-lasting changes are associated with tonic firing (1). Dopaminergic neurons exhibit both of these firing patterns. Phasic activity accompanies salient stimuli, whereas tonic firing regulates the steady-state extracellular concentration (2). For this reason, chemical sensors for dopamine should be able to operate on a wide range of time scales in behaving animals. Microdialysis, a commonly used in vivo chemical sampling technique, is well suited to measure the minute-to-minute changes (tonic) that occur after uptake inhibition by agents such as cocaine (3, 4). In vivo voltammetry, another approach for dopamine sampling, can measure much faster events, enabling phasic dopamine changes to be measured (5).
A limitation of all voltammetric techniques has been their chemical selectivity. Fast-scan cyclic voltammetry at carbon-fiber microelectrodes (6) provides rapid measurements and yields a chemical signature, the cyclic voltammogram, that allows distinction among electroactive molecules that are present in the brain. The electrode is highly sensitive to dopamine relative to dihydroxyphenylacetic acid and ascorbate, two major interferants, and the voltammogram of dopamine is distinct from those for a variety of neurochemical substances, although it is the same as that for norepinephrine (7). However, measurements in behaving rats have revealed that rapid dopamine changes are usually accompanied by other rapid changes in the electrochemical signal (5, 8, 9). Measurements with ion-selective electrodes demonstrated that these signals arise from a change in the pH of the brain extracellular fluid (10). Therefore, an objective method is needed to resolve the detected chemical events, assign them to specific compounds, and evaluate their temporal characteristics.
Voltammetric electrodes are similar to other multivariate chemical sensors (11-14), the signals of which arise from energy-dependent processes of molecules at the sensor surface. To unravel the potentially numerous chemical contributors, these sensors require sophisticated data processing. Although numerical deconvolution has been used with differential pulse voltammetric data to resolve dopamine, dihydroxyphenylacetic acid, and ascorbate (15), its use has been limited. Another powerful method that has been used with a number of sensor types is principal component regression. The method allows minimization of the recorded data set, feature extraction, and quantitative evaluation of chemical contributors and provides a way to minimize noise (16, 17). However, it has seen little use with electrochemically based in vivo sensors.
We recently demonstrated that principal component regression can be used with fast-scan cyclic voltammetric data to resolve multiple substances released from biological cells and in brain slices (18). In this article we describe the use of this method to extract the major components that comprise a series of background-subtracted cyclic voltammograms recorded in the brain of awake and unrestrained rats. This approach enables separation of the pH-related signal that accompanies dopamine release from stimulated neurons. Furthermore, the measurements on a subsecond time scale reveal that cocaine induces increases in extracellular dopamine that occur as cocaine reaches its molecular target in the brain.
Materials and Methods
Chemicals. Unless stated, all chemicals were purchased from Sigma-Aldrich (St. Louis) and used as received. Solutions were prepared by using doubly distilled deionized water (Megapure system, Corning). Tris buffer solution, pH 7.4 (15 mM Tris/126 mM NaCl/2.5 mM KCl/25 mM NaHCO3/2.4 mM CaCl2/1.2 mM NaH2PO4/1.2 mM MgCl2/2.0 mM Na2SO4), was used in calibrations. Stock solutions of analyte were prepared in 0.1 M HClO4, and dilute solutions were made in Tris buffer on the day of use.
Electrodes. Glass-encased carbon-fiber microelectrodes were constructed as described (19). Individual 5-μm-diameter carbon fibers (T-650 Thornell, Amoco Corp., Greenville, SC) were aspirated into glass capillaries (A-M Systems, Carlsborg, WA) and then pulled in a vertical micropipette puller (Narishige, Tokyo). The microelectrode was then inspected under an optical microscope. Electrodes having a good seal between the glass and the carbon fiber were cut with a scalpel to a length of 50-100 μm. All others were discarded. Before use, electrodes were soaked in 2-propanol purified with Norit A-activated carbon (ICN, Costa Mesa, CA) for at least 10 min (20). The reference electrodes were chloridized silver wires (0.5 mm diameter; Sigma-Aldrich) in 0.1 M HCl. All potentials reported are versus Ag/AgCl.
Data Acquisition. Voltammetric recordings were made with a triangular waveform (-0.4 to 1.3 V versus Ag/AgCl, 400 V/s) repeated every 100 ms. The electrode was held at -0.4 V between scans. The waveform was generated and the voltammetric signal was collected by using labview (National Instruments, Austin, TX) and a multifunction data-acquisition board (PCI-6052E, National Instruments). A PCI-6711E (National Instruments) board was used to synchronize waveform acquisition, data collection, and stimulation delivery (7). Waveform processing and current transduction used custom-built instrumentation (University of North Carolina, Department of Chemistry, Electronics Facility). Data were collected at 107 kHz, and the output signal was low-pass-filtered at 30 kHz before being digitized.
Animals and Surgery. Male Sprague-Dawley rats implanted with a jugular vein catheter (250-350 gauge; Charles River Laboratories, Wilmington, MA) were housed individually on a 12:12-h light/dark cycle (lights on at 7 a.m.) with ad libitum access to food and water. Three days before experiments, rats were anesthetized with ketamine hydrochloride (100 mg/kg, i.p.) and xylazine hydrochloride (20 mg/kg, i.p.) and placed in a stereotaxic frame for implantation of electrodes and cannula (21). A guide cannula (Bioanalytical Systems, West Lafayette, IN) was positioned above the nucleus accumbens (1.7 mm anterior, 0.8 mm lateral, -2.5 mm ventral relative to bregma). The Ag/AgCl reference electrode was placed contralateral to the guide cannula beneath the meningeal membrane, and both were secured in place with machine screws and cranioplastic cement. A carbon-fiber microelectrode was lowered through the guide cannula into the nucleus accumbens. A bipolar stimulating electrode (Plastics One, Roanoke, VA) was lowered into the substantia nigra/ventral tegmental area (5.2 mm posterior, 1.0 mm lateral, 7.5 mm ventral relative to bregma). The stimulating electrode was lowered until electrically evoked release was observed at the carbon-fiber microelectrode. An analog stimulus isolator (A-M Systems) delivered a biphasic (2 ms per phase), 60-pulse, 60-Hz, 125-μA stimulation. The carbon-fiber microelectrode was removed, and the stimulating electrode was secured in place. All procedures were performed in accordance with the University of North Carolina Animal Care and Use Committee.
Recording Sessions. On the experimental day, a carbon-fiber microelectrode was lowered into the nucleus accumbens with a microdrive (University of North Carolina, Department of Chemistry, Instrument Shop). The position of the microelectrode was optimized by monitoring electrically evoked (biphasic, 2 ms per phase, 24 pulses, 60 Hz, 125 μA) dopamine release. When a robust signal was obtained in the nucleus accumbens core, stimulated release was characterized at that location. During studies of cocaine, files (90 s in length) were collected continuously. After 15 min of baseline collection, saline was infused (i.v., 20 s per infusion), followed by 0.3 mg/kg, 1.0 mg/kg, and 3.0 mg/kg cocaine infusions at 15-min intervals. After the experimental session, the electrode was removed and calibrated with 1 μM dopamine and acidic and basic 0.1 pH unit changes in a flow-injection analysis system (19).
Data Analysis. After collection, background subtraction and digital filtering (4-pole Bessel filter, 2 kHz) were performed with locally written programs in labview (National Instruments). Color representation of the current was used to visualize the data (22) with the abscissa as time and the applied potential as the ordinate. A nonlinear color scale was used to facilitate visual recognition of the changes in current. Because the peak oxidation potential for dopamine is 0.65 V with the conditions used, changes in the current at this potential were examined. To evaluate the presence of dopamine, individual, background-subtracted voltammograms were compared to voltammograms for dopamine, and a correlation coefficient (r) was determined (23). Except for norepinephrine, the cyclic voltammograms of all substances tested have an r value of <0.86 when compared to dopamine (7). In this work, cyclic voltammograms were assigned to dopamine only if r > 0.86.
Principal component regression allows dopamine to be identified in the presence of interferences (18). It was performed by using matlab (Mathworks, Natick, MA). The number of factors kept in the model was selected so that >99.5% of the variance could be captured. The quality of the fit was evaluated by reproducing the data set with the regression values and evaluating the difference from the measured data. The difference between each point was squared, and the sum was taken for each cyclic voltammogram to yield a residual (Q). Fluctuations in the dopamine signal were considered concentration transients if they had a signal-to-noise ratio of >5. Their amplitude was determined with mini analysis (Synaptosoft, Decatur, GA), a program written to characterize transient events (24). An ANOVA was used to test statistical significance.
Results
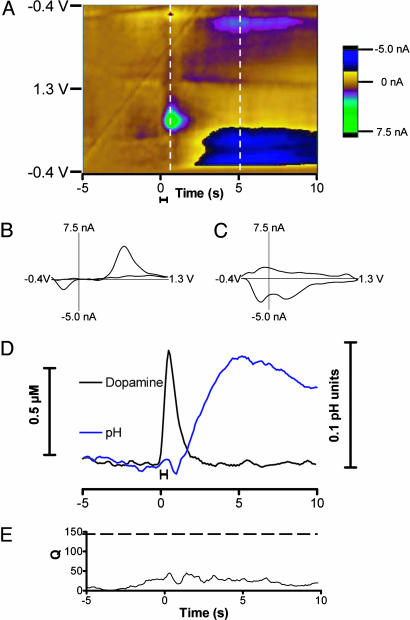
Dopamine Release and pH Changes Evoked by Impulse Flow in the Nucleus Accumbens. Electrical stimulation of dopaminergic cell bodies in the substantia nigra/ventral tegmental area evokes dopamine release at terminal regions as established by electrochemical and other procedures (25, 26). Fast-scan cyclic voltammetry was used to monitor chemical events in the nucleus accumbens during such stimulation. To remove the background and charging currents, the collected set of cyclic voltammograms was subtracted from the average of the first 10. Subtracted voltammograms then were encoded with the current in false color so that all the detected electrochemical changes in the measurement interval are visualized (Fig. 1A). During the stimulation, an increase in current at 0.65 V (the potential at which dopamine is oxidized) is observed. Another species changes in concentration after the dopamine disappears (with features at -0.25 and 0.20 V on the positive-going scan and at -0.10 V on the negative-going scan). The background-subtracted cyclic voltammogram recorded at the end of the stimulation (Fig. 1B) resembles one recorded in a solution containing dopamine, but it is not identical (r = 0.86). The background-subtracted cyclic voltammogram recorded at a later time (Fig. 1C) arises from changes in the residual current caused by a basic pH change that originates from a change in local blood flow (10).
Fig. 1.
Electrically evoked dopamine release. (A) Color representation of voltammograms obtained during electrical stimulation of dopaminergic neurons. The solid bar under the color plot reflects the duration of the stimulation (24 pulses at 60 Hz). The abscissa is time, and applied potential is plotted on the ordinate. Each successive cyclic voltammogram is plotted with the current shown in false color. (B) A cyclic voltammogram of dopamine taken at the end of the stimulation indicated by the first vertical line on the color plot. (C) A cyclic voltammogram of a basic change in pH taken 5 s after the stimulation at the time indicated by the second vertical line. (D) Changes in dopamine concentration and pH generated by principal component regression from the data shown in A.(E) The residual (Q) associated with the collected data.
In Vivo Training Set. With measurements in brain slices or at single cells, an in vitro training set was used to quantitate signals (18). However, there was poor correlation of the in vivo cyclic voltammograms with those obtained in vitro after in vivo use (average r = 0.8 ± 0.3; n = 6), and this necessitated an in vivo training procedure. The poor correlation may be a result of fouling of the electrode during removal from the brain. Another source of deviation is the Ag/AgCl reference electrode, the potential of which may shift with long-term implantation (27), and after 2-5 days, an offset of -0.2 V may be present. To account for this offset, the potential limits of the voltammetric waveform were adjusted, but a small voltage error may persist.
The in vivo training set was generated from background-subtracted cyclic voltammograms collected during stimulations by using different frequencies and number of pulses at the same location, which evoked different concentrations of dopamine at the end of each stimulation. Similarly, the training set for extracellular pH changes was obtained from the background-subtracted cyclic voltammograms 5-10 s after each stimulation. At least five voltammograms were obtained for each species from five stimulations (three 60-Hz and two 30-Hz stimulations comprising between 6 and 24 pulses). The current amplitude was converted to concentration units or pH values based on calibration of the electrode after the in vivo experiment. The background-subtracted cyclic voltammograms used in the training set were reduced by principal component analysis. Usually, only five factors were retained, which captured 99.5% of the variance in the training sets. The resulting eigenvectors were used with regression analysis to evaluate the stimulated electrochemical responses for dopamine and pH changes.
The analysis reveals that dopamine (Fig. 1D, black trace) increases during the stimulation and then is cleared from the extracellular space. A basic pH shift (Fig. 1D, blue trace) can be seen after the stimulation. The residual (Q; Fig. 1E) is below the 95% confidence limit indicated by the dashed line (28).
Various sources, mostly background drift, affect the residuals. Small changes in the background can easily contaminate the measurements of physiological concentrations of dopamine, which are often only 1% of the measured current. Transient electrical artifacts (i.e., arising during rapid movement of the animal) cause large, transient residuals, but they can be identified and the signals blanked out.
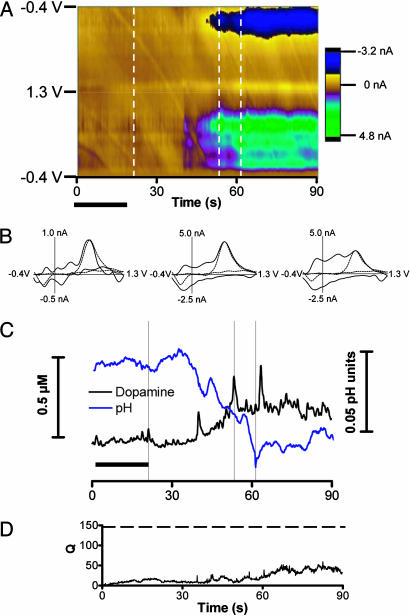
In Vivo Cocaine Infusions. Using the same training set, we evaluated the effect of i.v. cocaine infusions on extracellular dopamine in the nucleus accumbens. The time epoch examined was increased relative to the electrical stimulation to analyze the changes induced by cocaine. The electrochemical results during and after an infusion (20-s duration) of 0.3 mg/kg cocaine are shown in Fig. 2. Approximately 20 s after the end of infusion, the color representation of the voltammetric data (Fig. 2A) indicates the beginning of a change in electroactive species that appears to be the composite of multiple substances. Examination of individual background-subtracted cyclic voltammograms confirms this view (Fig. 2B; the vertical dashed lines indicate their collection time). A transient event 2 s after the end of the infusion exhibits a cyclic voltammogram that has dopaminergic features (Fig. 2B). However, the background-subtracted cyclic voltammograms obtained at times at which the robust color changes occur do not correlate well with dopamine (also Fig. 2B). Indeed, the background-subtracted cyclic voltammograms in this time period seem to comprise different components.
Fig. 2.
Voltammetric changes after an i.v. cocaine infusion. (A) Color representation of voltammograms obtained after an i.v. infusion of cocaine (0.3 mg/kg). The infusion time is denoted by the solid horizontal bars beneath the color plot. Data are plotted as described for Fig. 1. (B) Cyclic voltammograms recorded at 22, 52, and 61 s after the infusion (times are indicated by the vertical bars). The cyclic voltammograms represented with the dashed lines are those obtained with electrical stimulation. The correlation coefficients (r) for the voltammograms with that recorded during electrical stimulation are 0.83, 0.84, and 0.61, respectively. (C) Changes in dopamine concentration and pH generated by principal component regression. (D) The residual (Q) associated with the collected data.
Analysis of the data by principal component regression with data obtained during electrical stimulation experiments (the training set) allows resolution of dopamine and pH changes (Fig. 2C). Dopamine increases ≈20 s after the end of the infusion and gradually reaches a new plateau concentration. Superimposed on the plateau are pronounced dopamine transients that are larger than those observed during the infusion. Almost simultaneously, the pH becomes more acidic and also reaches a plateau. The residual (Fig. 2D) remains well below the 95% confidence limit for the entire 90 s measurement interval. Thus, although multiple electroactive substances are undoubtedly changing, the major contributors are dopamine and pH.
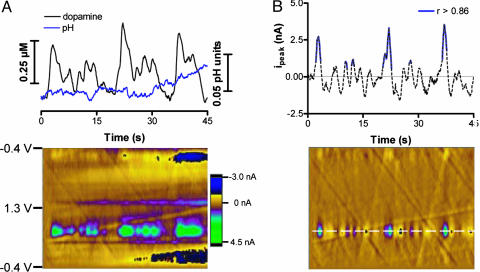
Resolution of Rapid Dopamine Concentration Transients After Cocaine. As we have reported previously, large dopamine transients occur in the nucleus accumbens after cocaine (29). These seem to originate from the inhibition of the dopamine transporter, because they also occur after another uptake inhibitor, nomifensine (30). These transients were characterized previously with a differential method: instead of subtracting a set of voltammograms recorded at a fixed time, the cyclic voltammograms measured 1 s earlier were subtracted from the cyclic voltammogram at each time point (29-31). This moving subtraction was applied to the whole set, generating an array that also can be examined in false color and from which individual cyclic voltammograms can be evaluated.
Because this procedure gives a differential of the original data, it masks slow changes of dopamine as shown in Fig. 2, but it allows rapid ones to be resolved. A comparison of this method with the principal component method for data recorded 70 s after the end of the infusion of 1.0 mg/kg cocaine is shown in Fig. 3. The color representation of the background-subtracted cyclic voltammograms (Fig. 3A Lower) shows several transient events at the potential at which dopamine is oxidized. Principal component regression of these data reveals that both dopamine and pH change during the measurement interval (Fig. 3A Upper). From 90 to 135 s, the pH remains more acidic and begins to return to its initial value. In the 45-s interval shown, 14 dopamine concentration transients were found with a mean concentration of 120 ± 20 nM (±SEM). Analysis of the data in Fig. 3A with the differential method leads to a color representation (Fig. 3B Lower) that enhances the transient events. The current at the potential for dopamine oxidation (Fig. 3B Upper) was evaluated with the cyclic voltammetry template method, but only the portions of the signal shown in solid blue met the criterion to be dopamine (r > 0.86). Over the same 45-s interval, only 6 dopamine transients were identified, which is lower than determined with the principal component method. Their mean concentration (190 ± 30 nM) was greater because small transient events were not detected.
Fig. 3.
Dopamine concentration transients after cocaine. (A) Results obtained with principal component regression. (Upper) Changes in pH and dopamine concentration. (Lower) Two-dimensional color representation of the background-subtracted cyclic voltammograms collected 90 s after cocaine (1.0 mg/kg) infusion. (B) Results from the same data as shown in A obtained with the differential method. The current at 0.65 V (Upper) is plotted versus time; the solid blue line indicates where dopamine was identified (r > 0.86). (Lower) Two-dimensional color representation of the cyclic voltammograms after the differential method was applied.
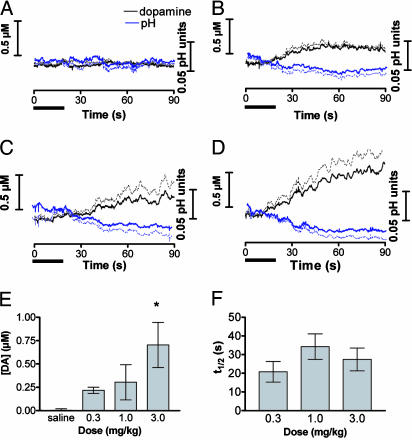
Resolution of Tonic Dopamine Changes After Different Doses of Cocaine. To examine the dose-response of dopamine to cocaine, infusions (20 s in duration) of saline and three successive, cumulative doses (0.3, 1.0, and 3.0 mg/kg) of cocaine were given at 15-min intervals in six animals. The mean results for dopamine, after principal component regression of the cyclic voltammetric data, are shown in Fig. 4. After saline, the dopamine concentration did not change. An increase in extracellular dopamine concentration was seen with all three doses (Fig. 4 A-D) that progressed during the 90-s measurement interval. The traces resemble the one shown in Fig. 2C except that the averaging process removes the randomly occurring, fast dopamine concentration changes. Cocaine treatment significantly increased the extracellular dopamine concentration [Fig. 4E; one-way ANOVA, F(3,20) = 5.5; P < 0.01]. The time to reach half-maximal concentration was similar for each dose (Fig. 4F). The error associated with the measurements is caused by individual variation in the magnitude of change in dopamine concentration. All the traces comprising Fig. 4 had residuals that were less than the limit set for 95% confidence. However, attempts to analyze the data over time intervals longer than 90 s gave residuals that exceeded this value in many instances, mainly due to alterations in the background on this time scale.
Fig. 4.
Dopamine changes caused by i.v. cocaine infusion. Rats were given saline and cocaine in cumulative doses (n = 6 rats). Error bars represent SEM. The solid bar beneath each graph indicates the infusion time. (A-D) Average time course of dopamine (black lines) and pH (blue lines) changes for saline (A) and 0.3 (B), 1.0 (C), and 3.0 (D) mg/kg cocaine. The dashed lines above the dopamine trace and below the pH trace represent the SEM. (E) Average changes in dopamine concentration measured for 10 s beginning 60 s after the infusion (one-way ANOVA, Duncan post hoc test: *, P < 0.05 versus saline). (F) Time for dopamine concentration to reach one half of its maximum value after cocaine infusion.
Acidic pH changes were also observed after each dose of cocaine (Fig. 4). These changes often coincided with the increases in dopamine (Figs. 2 and 4). Regulation of extracellular pH is a dynamic process and depends on many factors including metabolism and blood flow (10). Cocaine is a vasoconstrictor (32), impeding blood flow and the removal of carbon dioxide, making the local environment more acidic. Failure to separate these acidic pH changes from dopamine may account for differences between previous studies and the data presented here (33, 34). Measurements performed with both cyclic voltammetry (35) and chronoamperometry (36) have been shown to be sensitive to changes in pH.
Discussion
The data obtained with principal component regression of voltammograms from carbon-fiber microelectrodes provides an unprecedented view of neurotransmitter concentration fluctuations in the brain of a freely moving animal. The fast-scan cyclic voltammograms contain information about several electrochemical phenomena. These include the electrode-charging current, the electrolysis of carbon-surface functional groups, the pH of the solution adjacent to the carbon surface, and the electrolysis of solution species adjacent to the electrode (7). Background subtraction removes the components that remain constant during the measurement interval and is necessary because of the large amplitude of the surface-associated processes. The background-subtracted cyclic voltammograms contain information from local pH and dopamine fluctuations (10). Principal component regression provides an objective way to separate and evaluate these two contributors. The data after cocaine infusion analyzed with this approach provide the first experimental demonstration that this drug causes a tonic increase in dopamine concentration with dopamine concentration transients superimposed on it while simultaneously causing acidification of the extracellular fluid.
Because in vivo electrochemical experiments have classically suffered from poor chemical resolution, they have often been designed with simple, experimenter-controlled stimuli such as electrical stimulation (26, 37), transient exposure to high K+ (38), or exogenously applied neurotransmitters (39). The current at the potential at which the species of interest is electrolyzed provides the time course of the chemical changes. In such experiments, a simple chemical identifier such as a single cyclic voltammogram obtained at the maximal response is sufficient because the observation time is easily constrained. The basis for our in vivo training set is the prior studies with electrical stimulation of dopaminergic fibers or cell bodies that established by a variety of means that dopamine is the only electroactive compound detectable at the end of stimulation (25, 26) and that a basic pH change occurs a few seconds later (10). Chemical changes during behavioral or pharmacological challenges in alert animals arise from more complex origins, and the chemical identity at each time point needs to be established.
To estimate the correctness of the chemical identity extracted with principal component regression, we compared its results with those obtained by our previous approaches. For example, the color plot (Fig. 1 A) clearly reveals that dopamine is released during the stimulation interval and that this is followed by a basic pH change. These are exactly the results returned from the regression analysis (Fig. 1D). Similarly, after cocaine, the color plots and the differential method clearly indicate that changes in dopamine occur (Figs. 2 and 3) and that they are accompanied by extracellular acidification. However, only principal component regression provides the capability for continuous resolution of dopamine and pH changes. Although the use of in vivo training set limits the number of components that can be distinguished, it does not limit the analysis. After cocaine challenge, the residuals show that the fit is good when only dopamine and pH are considered, although the substances that change are different from those during electrical stimulation. This is because dopamine and pH changes are the major contributors to the signal with both stimuli. The results clearly reveal two of the advantages of the principal component method: it not only provides an objective measure of dopamine (the residuals revealed that all the assignments were valid at the 95% confidence level over the time intervals examined), but it also improves the signal-to-noise ratio, allowing smaller dopamine concentration transients to be discerned. Furthermore, unlike the differential method, the temporal changes in dopamine concentration are preserved.
Conditioned behaviors typically occur on a time scale of a few seconds. Thus, to monitor the chemical and physiological responses of neurons during such behaviors, subsecond measurements are required. Single-unit electrophysiological recordings provide an instantaneous measure of neuronal activity and have revealed the role of neural pathways in specific behaviors (40). Chemical sensing of neurotransmitters in the brain of behaving animals is now feasible on a similar time scale. Transient increases in dopamine, identified with the differential method, have been observed previously during various reward-based behaviors including introduction of conspecifics (8), cocaine seeking (5), and food seeking (9). Pharmacological treatments with the CB1 receptor agonist, WIN55212-2 (31), or the dopamine transporter inhibitors, nomifensine (30) and cocaine (29), also elicit increases in dopamine concentration transients (Fig. 3).
As well as allowing resolution of fast dopamine changes, the principal component regression also allows resolution of slower changes in dopamine that are often masked by concurrent pH changes. After i.v. cocaine delivery, the voltammetric signal is comprised of at least two distinct components. Because cocaine inhibits the dopamine transporter, the primary means for removing extracellular dopamine, a tonic change in dopamine concentration is anticipated. Indeed, by using 1-min sampling, dopamine has been shown to increase by in vivo microdialysis after 2 mg/kg cocaine self-administered by rats with similar infusion times (3). As in the present study, dopamine was clearly elevated 90 s after the initiation of the infusion. However, the higher temporal resolution of the voltammetric data allows the time course to be more clearly discerned. For example, at the highest dose tested, dopamine increases during the time of the infusion (Fig. 4). By using changes in the uptake rate after electrically stimulated release, others have shown that cocaine reaches the brain and exerts its effects on dopamine uptake within 10 s of its administration (41, 42).
Although the microdialysis and voltammetric techniques provide comparable results on the time course of the effect of cocaine on extracellular dopamine, the amplitude of tonic concentration changes are dramatically different. By using the no-net-flux method, microdialysis measurements estimate this basal dopamine concentration in the nucleus accumbens at ≈5 nM (43). The voltammetric approach requires background subtraction, which precludes direct measurement of the basal concentration. However, the changes in dopamine concentration after i.v. cocaine exceed 700 nM at the highest cocaine dose. In contrast, microdialysis results report increases of ≈8-fold, which would correspond to 40 nM extracellular dopamine (3), a value more than an order of magnitude less than the current estimate. Although disturbances of the tissue with either probe (44-46) or errors in calibration may account for this large difference, differences in sampling region are also a likely factor. The carbon-fiber electrode is ≈40-fold shorter and 50-fold smaller in diameter than typical microdialysis probes. Electrically evoked dopamine release has been reported to vary over dimensions similar to the carbon-fiber microelectrode (6). The larger-sized microdialysis probes sample regions of both high and low density of dopamine terminals, whereas the voltammetric measurements in this study were obtained at locations that supported robust stimulated dopamine. This would result in higher concentrations sampled by the electrode but also an increased error in the mean responses because of the high sensitivity to local density differences. The intermediate dose of cocaine (1.0 mg/kg) induced increases in dopamine concentration of 300 ± 170 nM. When extracellular dopamine concentrations have been modeled at an equivalent dose of cocaine, changes on the same order of magnitude as those detected in this work resulted (1).
Principal component regression of fast-scan cyclic voltammetric data collected in vivo reveals rapid dopamine concentration transients as well as slower changes. Training with a set obtained in vivo allows dissociation of dopamine concentration changes from accompanying pH changes. However, as we have shown previously (18), the method does have limitations. It misassigns ≈5% of the signal because of voltammetric shifts and noise inherent to fast-scan cyclic voltammetry. Finally, background subtraction that is required with fast-scan cyclic voltammetry imposes a limit on the usefulness of the method at present for physiological concentrations that occur over periods longer than ≈90 s.
Acknowledgments
We thank the University of North Carolina, Department of Chemistry, Electronics Facility, for the instrumentation used in this work. This research was supported by National Institute of Neurological Disorders and Stroke Grant NS15841.
Author contributions: M.L.A.V.H. and R.M.W. designed research; M.L.A.V.H., A.S.K., J.L.A., J.F.C., P.E.M.P., and K.M.W. performed research; M.L.A.V.H. and A.S.K. analyzed data; and M.L.A.V.H. and R.M.W. wrote the paper.
References
- 1.Venton, B. J., Zhang, H., Garris, P. A., Phillips, P. E., Sulzer, D. & Wightman, R. M. (2003) J. Neurochem. 87, 1284-1295. [DOI] [PubMed] [Google Scholar]
- 2.Schultz, W. (1998) J. Neurophysiol. 80, 1-27. [DOI] [PubMed] [Google Scholar]
- 3.Wise, R. A., Newton, P., Leeb, K., Burnette, B., Pocock, D. & Justice, J. B., Jr. (1995) Psychopharmacology (Berl) 120, 10-20. [DOI] [PubMed] [Google Scholar]
- 4.Rouge-Pont, F., Usiello, A., Benoit-Marand, M., Gonon, F., Piazza, P. V. & Borrelli, E. (2002) J. Neurosci. 22, 3293-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips, P. E., Stuber, G. D., Heien, M. L., Wightman, R. M. & Carelli, R. M. (2003) Nature 422, 614-618. [DOI] [PubMed] [Google Scholar]
- 6.Robinson, D. L., Venton, B. J., Heien, M. L. & Wightman, R. M. (2003) Clin. Chem. 49, 1763-1773. [DOI] [PubMed] [Google Scholar]
- 7.Heien, M. L. A. V., Phillips, P. E. M., Stuber, G. D., Seipel, A. T. & Wightman, R. M. (2003) Analyst. 128, 1413-1419. [DOI] [PubMed] [Google Scholar]
- 8.Robinson, D. L., Heien, M. L. & Wightman, R. M. (2002) J. Neurosci. 22, 10477-10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roitman, M. F., Stuber, G. D., Phillips, P. E., Wightman, R. M. & Carelli, R. M. (2004) J. Neurosci. 24, 1265-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venton, B. J., Michael, D. J. & Wightman, R. M. (2003) J. Neurochem. 84, 373-381. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, L., Small, G. W. & Arnold, M. A. (2002) Anal. Chem. 74, 4097-4108. [DOI] [PubMed] [Google Scholar]
- 12.Potyrailo, R. A. & Hieftje, G. M. (1998) Anal. Chem. 70, 3407-3412. [DOI] [PubMed] [Google Scholar]
- 13.Epstein, J. R. & Walt, D. R. (2003) Chem. Soc. Rev. 32, 203-214. [DOI] [PubMed] [Google Scholar]
- 14.Frank, C. J., McCreery, R. L. & Redd, D. C. (1995) Anal. Chem. 67, 777-783. [DOI] [PubMed] [Google Scholar]
- 15.Mas, M., Fumero, B. & González-Mora, J. L. (1995) Behav. Brain Res. 71, 69-79. [DOI] [PubMed] [Google Scholar]
- 16.Lavine, B. K. & Workman, J., Jr. (2002) Anal. Chem. 74, 2763-2769. [DOI] [PubMed] [Google Scholar]
- 17.Kramer, R. (1998) Chemometric Techniques for Quantitative Analysis (Marcel-Dekker, New York).
- 18.Heien, M. L., Johnson, M. A. & Wightman, R. M. (2004) Anal. Chem. 76, 5697-5704. [DOI] [PubMed] [Google Scholar]
- 19.Kawagoe, K. T., Zimmerman, J. B. & Wightman, R. M. (1993) J. Neurosci. Methods 48, 225-240. [DOI] [PubMed] [Google Scholar]
- 20.Bath, B. D., Michael, D. J., Trafton, B. J., Joseph, J. D., Runnels, P. L. & Wightman, R. M. (2000) Anal. Chem. 72, 5994-6002. [DOI] [PubMed] [Google Scholar]
- 21.Phillips, P. E., Robinson, D. L., Stuber, G. D., Carelli, R. M. & Wightman, R. M. (2003) Methods Mol. Med. 79, 443-464. [DOI] [PubMed] [Google Scholar]
- 22.Michael, D., Travis, E. R. & Wightman, R. M. (1998) Anal. Chem. 70, 586A-592A. [DOI] [PubMed] [Google Scholar]
- 23.Troyer, K. P., Heien, M. L., Venton, B. J. & Wightman, R. M. (2002) Curr. Opin. Chem. Biol. 6, 696-703. [DOI] [PubMed] [Google Scholar]
- 24.Sombers, L. A., Hanchar, H. J., Colliver, T. L., Wittenberg, N., Cans, A., Arbault, S., Amatore, C. & Ewing, A. G. (2004) J. Neurosci. 24, 303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhr, W. G., Ewing, A. G., Caudill, W. L. & Wightman, R. M. (1984) J. Neurochem. 43, 560-569. [DOI] [PubMed] [Google Scholar]
- 26.Gonon, F. G. (1988) Neuroscience 24, 19-28. [DOI] [PubMed] [Google Scholar]
- 27.Zhang, X. J., Wang, J., Ogorevc, B. & Spichiger, U. E. (1999) Electroanalysis 11, 945-949. [Google Scholar]
- 28.Jackson, J. E. & Mudholkar, G. S. (1979) Technometrics 21, 341-349. [Google Scholar]
- 29.Stuber, G. D., Roitman, M. F., Phillips, P. E., Carelli, R. M. & Wightman, R. M. (2005) Neuropsychopharmacology 30, 853-863. [DOI] [PubMed] [Google Scholar]
- 30.Robinson, D. L. & Wightman, R. M. (2004) J. Neurochem. 90, 894-903. [DOI] [PubMed] [Google Scholar]
- 31.Cheer, J. F., Wassum, K. M., Heien, M. L., Phillips, P. E. & Wightman, R. M. (2004) J. Neurosci. 24, 4393-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albuquerque, M. L. & Kurth, C. D. (1993) Eur. J. Pharmacol. 249, 215-220. [DOI] [PubMed] [Google Scholar]
- 33.Gratton, A. & Wise, R. A. (1994) J. Neurosci. 14, 4130-4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiyatkin, E. A. & Stein, E. A. (1995) Neuroscience 64, 599-617. [DOI] [PubMed] [Google Scholar]
- 35.Wightman, R. M. & Robinson, D. L. (2002) J. Neurochem. 82, 721-735. [DOI] [PubMed] [Google Scholar]
- 36.Gerhardt, G. A. & Hoffman, A. F. (2001) J. Neurosci. Methods 109, 13-21. [DOI] [PubMed] [Google Scholar]
- 37.Wightman, R. M., Amatore, C., Engstrom, R. C., Hale, P. D., Kristensen, E. W., Kuhr, W. G. & May, L. J. (1988) Neuroscience 25, 513-523. [DOI] [PubMed] [Google Scholar]
- 38.Gerhardt, G. A., Cass, W. A., Hudson, J., Henson, M., Zhang, Z., Ovadia, A., Hoffer, B. J. & Gash, D. M. (1996) J. Neurochem. 66, 579-588. [DOI] [PubMed] [Google Scholar]
- 39.Frazer, A. & Daws, L. C. (1998) Ann. N.Y. Acad. Sci. 861, 217-229. [DOI] [PubMed] [Google Scholar]
- 40.Carelli, R. M. & Wightman, R. M. (2004) Curr. Opin. Neurobiol. 14, 763-768. [DOI] [PubMed] [Google Scholar]
- 41.Mateo, Y., Budygin, E. A., Morgan, D., Roberts, D. C. & Jones, S. R. (2004) Eur. J. Neurosci. 20, 2838-2842. [DOI] [PubMed] [Google Scholar]
- 42.Samaha, A. N., Mallet, N., Ferguson, S. M., Gonon, F. & Robinson, T. E. (2004) J. Neurosci. 24, 6362-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsons, L. H. & Justice, J. B., Jr. (1992) J. Neurochem. 58, 212-218. [DOI] [PubMed] [Google Scholar]
- 44.Yang, H., Peters, J. L., Allen, C., Chern, S. S., Coalson, R. D. & Michael, A. C. (2000) Anal. Chem. 72, 2042-2049. [DOI] [PubMed] [Google Scholar]
- 45.Bungay, P. M., Newton-Vinson, P., Isele, W., Garris, P. A. & Justice, J. B. (2003) J. Neurochem. 86, 932-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters, J. L., Miner, L. H., Michael, A. C. & Sesack, S. R. (2004) J. Neurosci. Methods 137, 9-23. [DOI] [PubMed] [Google Scholar]