Abstract
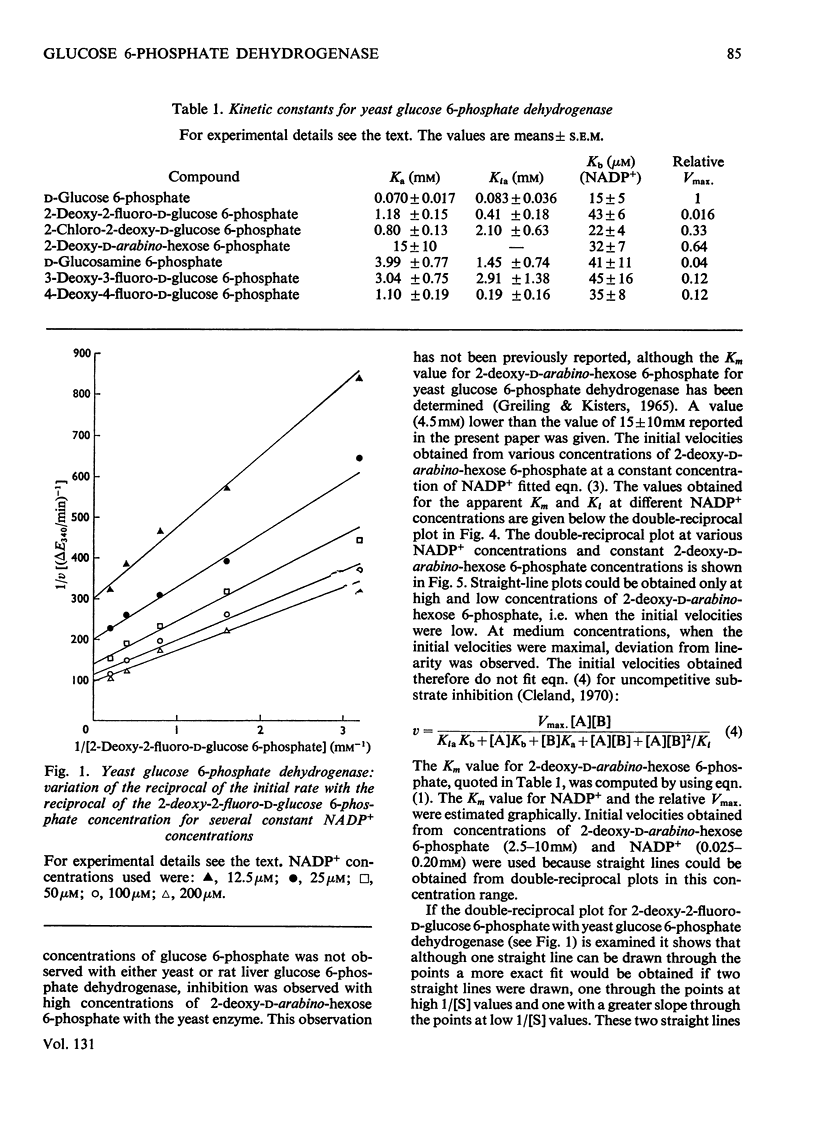
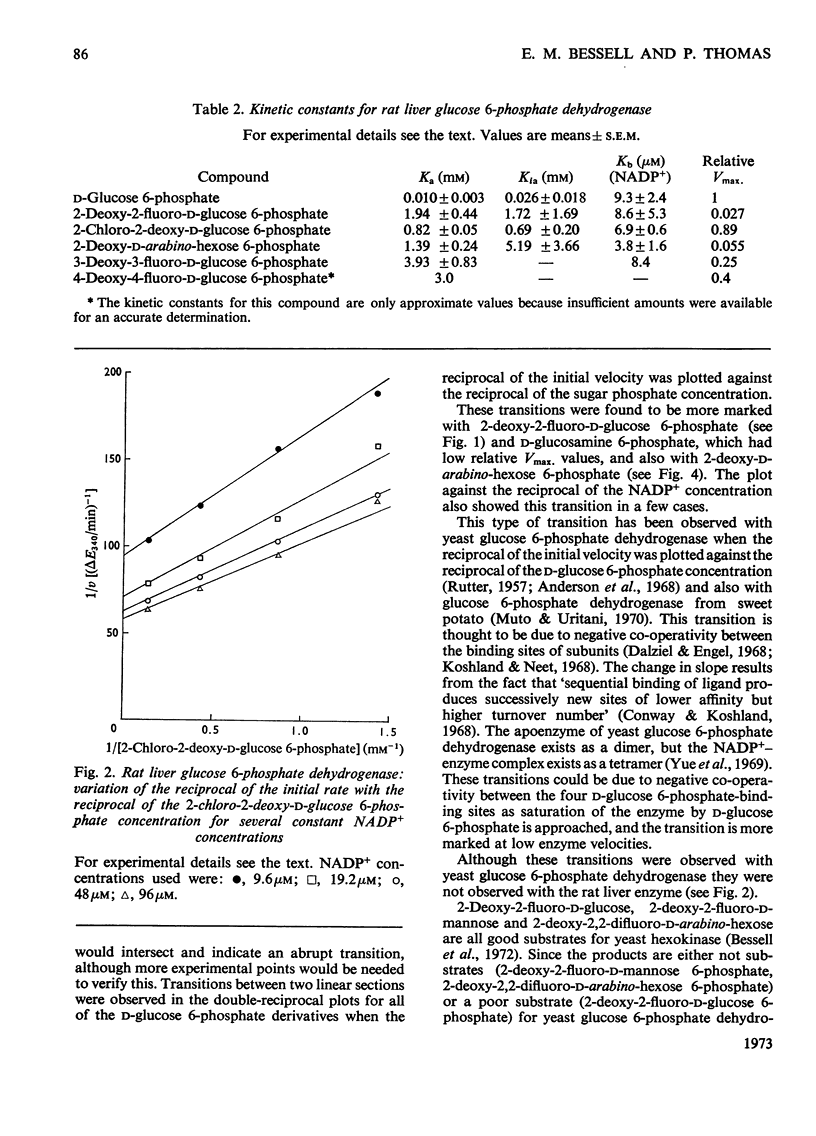
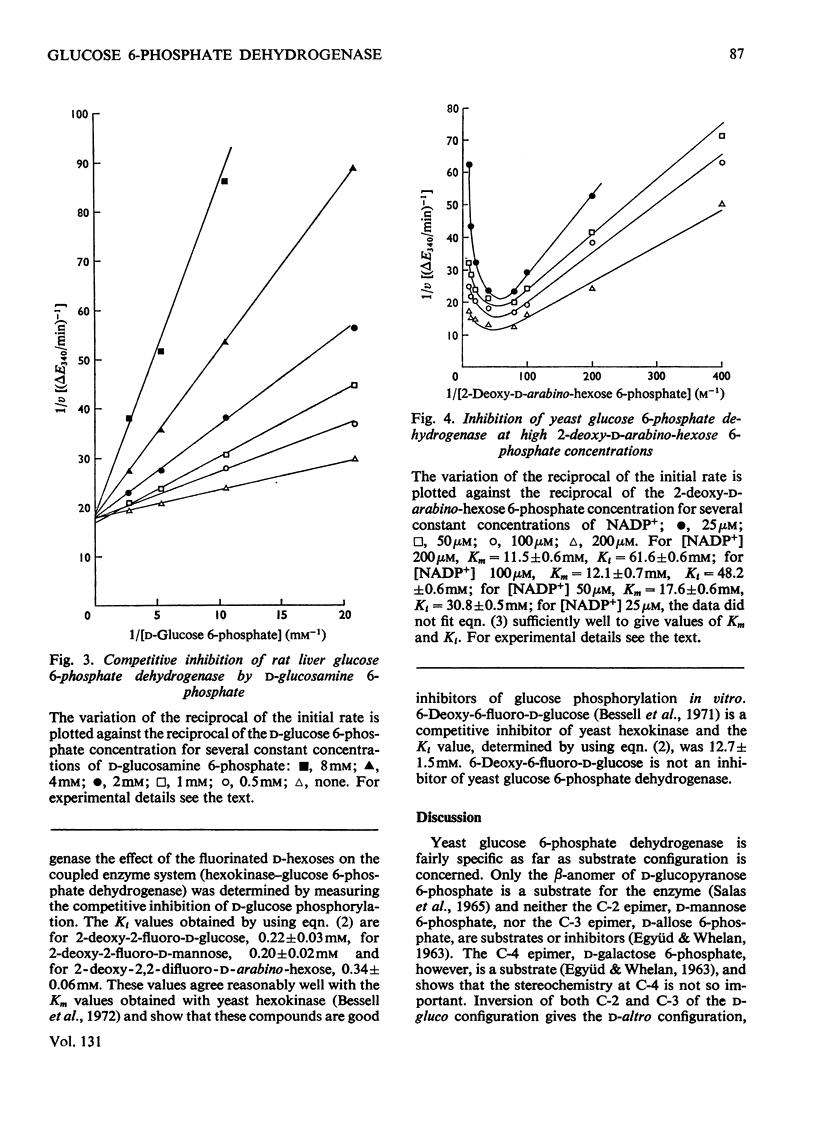
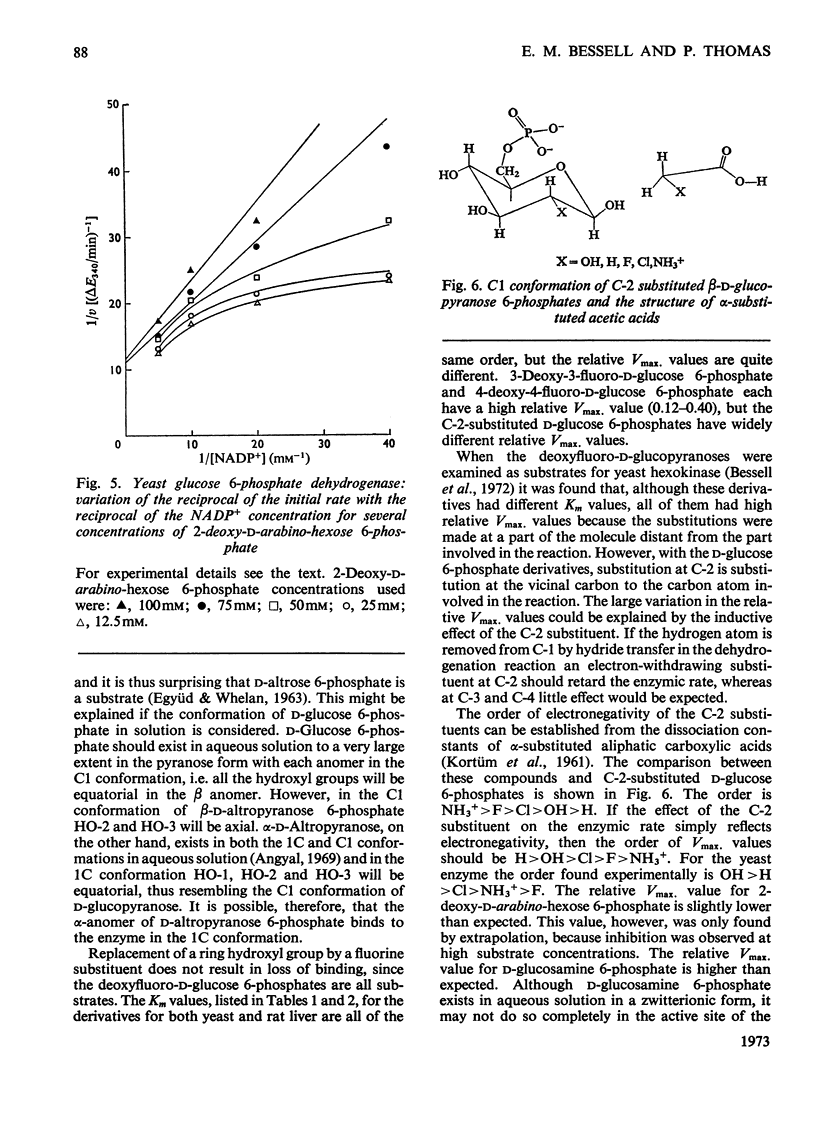
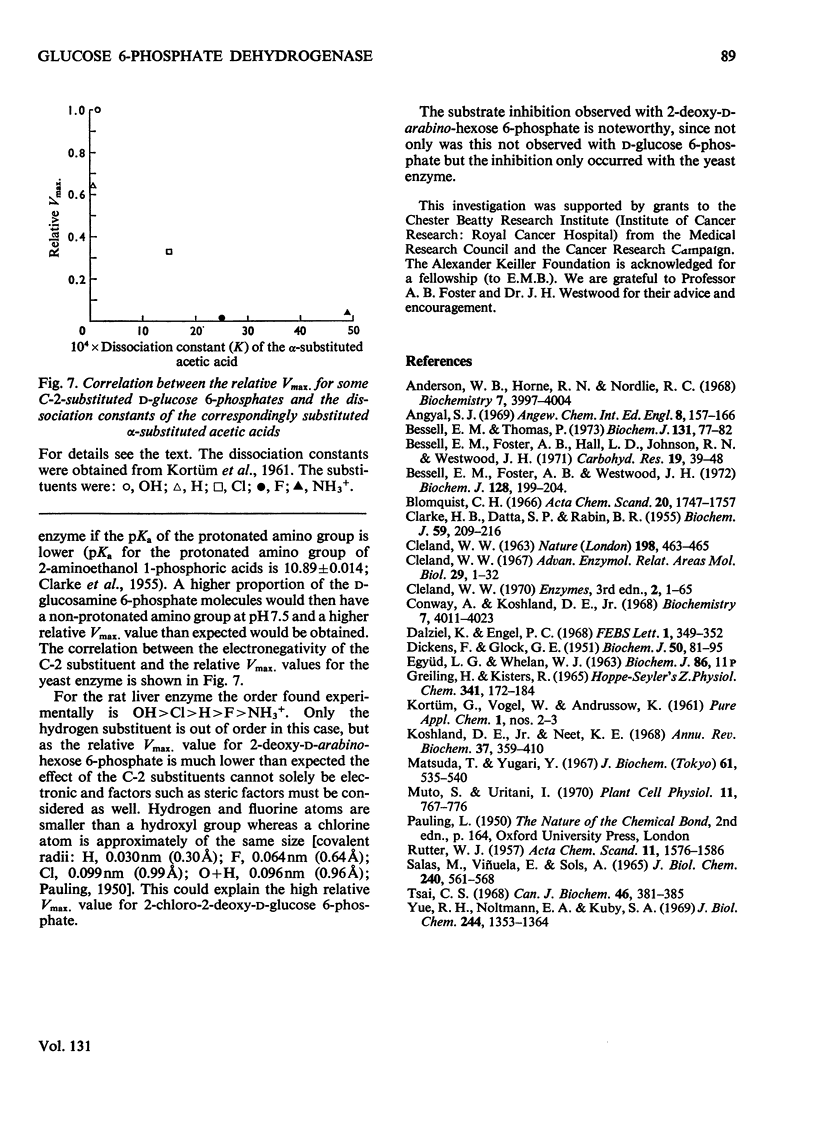
1. The deoxyfluoro-d-glucopyranose 6-phosphates are substrates for both yeast and rat liver glucose 6-phosphate dehydrogenase. 2. The Vmax. values (relative to d-glucose 6-phosphate) were determined for a series of d-glucose 6-phosphate derivatives substituted at C-2. The Vmax. values decreased with increasing electronegativity of the C-2 substituent. This is consistent with a mechanism involving hydride-ion transfer. 3. 2-Deoxy-d-arabino-hexose 6-phosphate (2-deoxy-d-glucose 6-phosphate) showed substrate inhibition with the yeast enzyme but not with the rat liver enzyme. 4. 2-Amino-2-deoxy-d-glucose 6-phosphate (d-glucosamine 6-phosphate) was a substrate for the yeast enzyme but a competitive inhibitor for the rat liver enzyme. 5. Lineweaver–Burk plots for the d-glucose 6-phosphate derivatives with yeast glucose 6-phosphate dehydrogenase were biphasic.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Horne R. N., Nordlie R. C. Glucose dehydrogenase activity of yeast glucose 6-phosphate dehydrogenase. II. Kinetic studies of the mode of activation by bicarbonate, phosphate, and sulfate. Biochemistry. 1968 Nov;7(11):3997–4004. doi: 10.1021/bi00851a029. [DOI] [PubMed] [Google Scholar]
- Bessell E. M., Foster A. B., Westwood J. H. Fluorinated carbohydrates. XI. 6-Deoxy-6-fluoro-D-glucose: an improved synthesis and the glycosyl fluoride derivatives. Carbohydr Res. 1971 Aug;19(1):39–48. doi: 10.1016/s0008-6215(00)80310-9. [DOI] [PubMed] [Google Scholar]
- Bessell E. M., Foster A. B., Westwood J. H. The use of deoxyfluoro-D-glucopyranoses and related compounds in a study of yeast hexokinase specificity. Biochem J. 1972 Jun;128(2):199–204. doi: 10.1042/bj1280199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessell E. M., Thomas P. The deoxyfluoro-D-glucopyranose 6-phosphates and their effect on yeast glucose phosphate isomerase. Biochem J. 1973 Jan;131(1):77–82. doi: 10.1042/bj1310077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomquist C. H. Electronic effects in horse liver alcohol dehydrogenase catalysis. Acta Chem Scand. 1966;20(7):1747–1757. doi: 10.3891/acta.chem.scand.20-1747. [DOI] [PubMed] [Google Scholar]
- CLARKE H. B., DATTA S. P., RABIN B. R. Thermodynamic quantities for the dissociation equilibria of biologically important compounds. 5. The second and dissociation of 2-aminoethanol 1-phosphoric acid. Biochem J. 1955 Feb;59(2):209–218. doi: 10.1042/bj0590209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. Computer programmes for processing enzyme kinetic data. Nature. 1963 May 4;198:463–465. doi: 10.1038/198463a0. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- DICKENS F., GLOCK G. E. Direct oxidation of glucose-6-phosphate, 6-phosphogluconate and pentose-5-phosphates by enzymes of animal origin. Biochem J. 1951 Nov;50(1):81–95. doi: 10.1042/bj0500081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel Keith, Engel Paul C. Antagonistic homotropic interactions as a possible explanation of coenzyme activation of glutamate dehydrogenase. FEBS Lett. 1968 Oct;1(5):349–352. doi: 10.1016/0014-5793(68)80153-x. [DOI] [PubMed] [Google Scholar]
- Greiling H., Kisters R. Untersuchungen über die Substratspezifität der Glucose-6-phosphat-Dehydrogenase. Hoppe Seylers Z Physiol Chem. 1965;341(4):172–184. [PubMed] [Google Scholar]
- HERS H. G. alpha-Glucosidase deficiency in generalized glycogenstorage disease (Pompe's disease). Biochem J. 1963 Jan;86:11–16. doi: 10.1042/bj0860011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Neet K. E. The catalytic and regulatory properties of enzymes. Annu Rev Biochem. 1968;37:359–410. doi: 10.1146/annurev.bi.37.070168.002043. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Yugari Y. Glucose-6-phosphate dehydrogenase from rat liver. I. Crystallization and properties. J Biochem. 1967 May;61(5):535–540. doi: 10.1093/oxfordjournals.jbchem.a128583. [DOI] [PubMed] [Google Scholar]
- SALAS M., VINUELA E., SOLS A. SPONTANEOUS AND ENZYMATICALLY CATALYZED ANOMERIZATION OF GLUCOSE 6-PHOSPHATE AND ANOMERIC SPECIFICITY OF RELATED ENZYMES. J Biol Chem. 1965 Feb;240:561–568. [PubMed] [Google Scholar]
- Tsai C. S. Relative reactivities of primary alcohols as substrates of liver alcohol dehydrogenase. Can J Biochem. 1968 Apr;46(4):381–385. doi: 10.1139/o68-056. [DOI] [PubMed] [Google Scholar]
- Yue R. H., Noltmann E. A., Kuby S. A. Glucose 6-phosphate dehydrogenase from brewers' yeast (Zwischenferment). 3. Studies on the subunit structure and on the molecular association phenomenon induced by triphosphopyridine nucleotide. J Biol Chem. 1969 Mar 10;244(5):1353–1364. [PubMed] [Google Scholar]


