Abstract
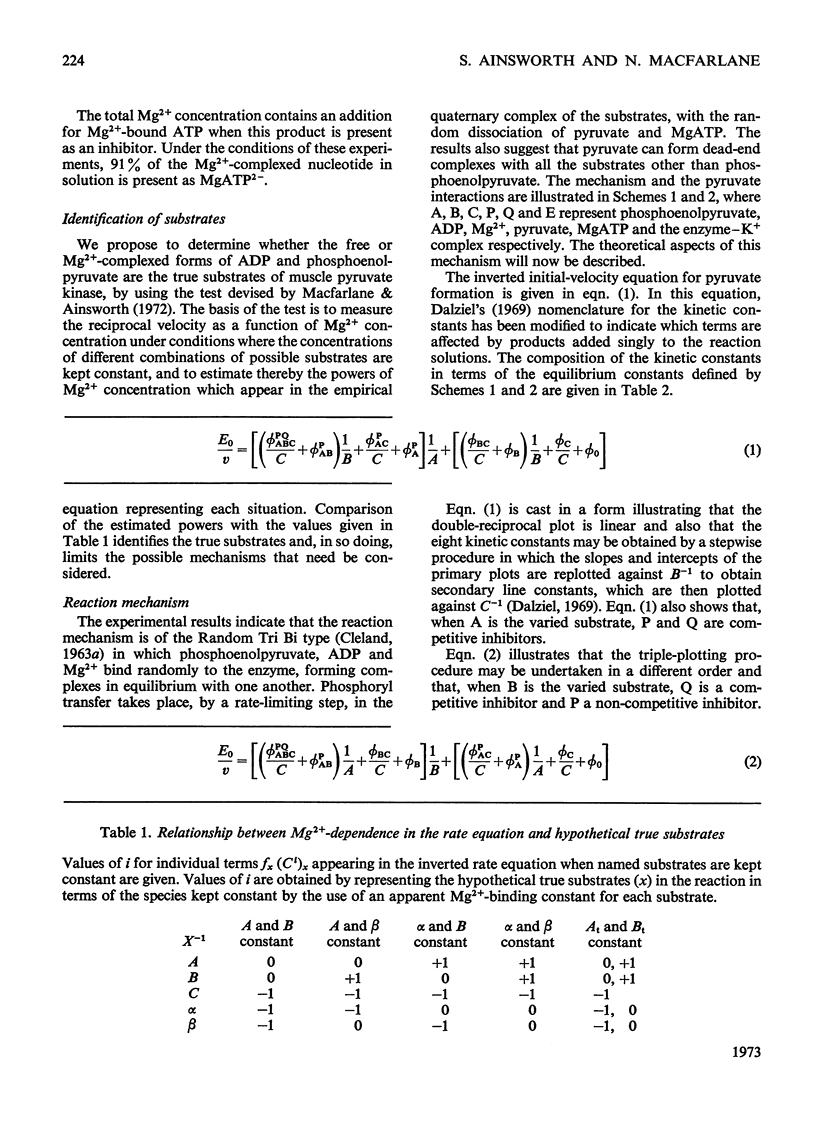
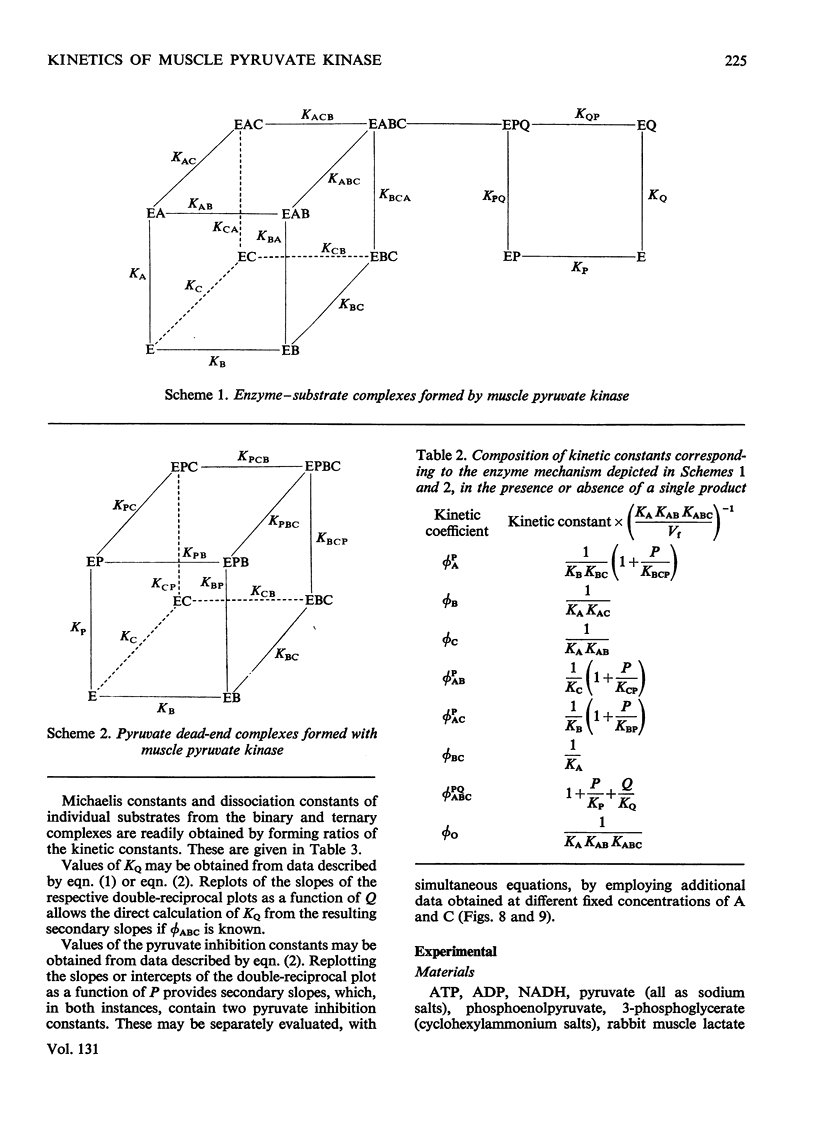
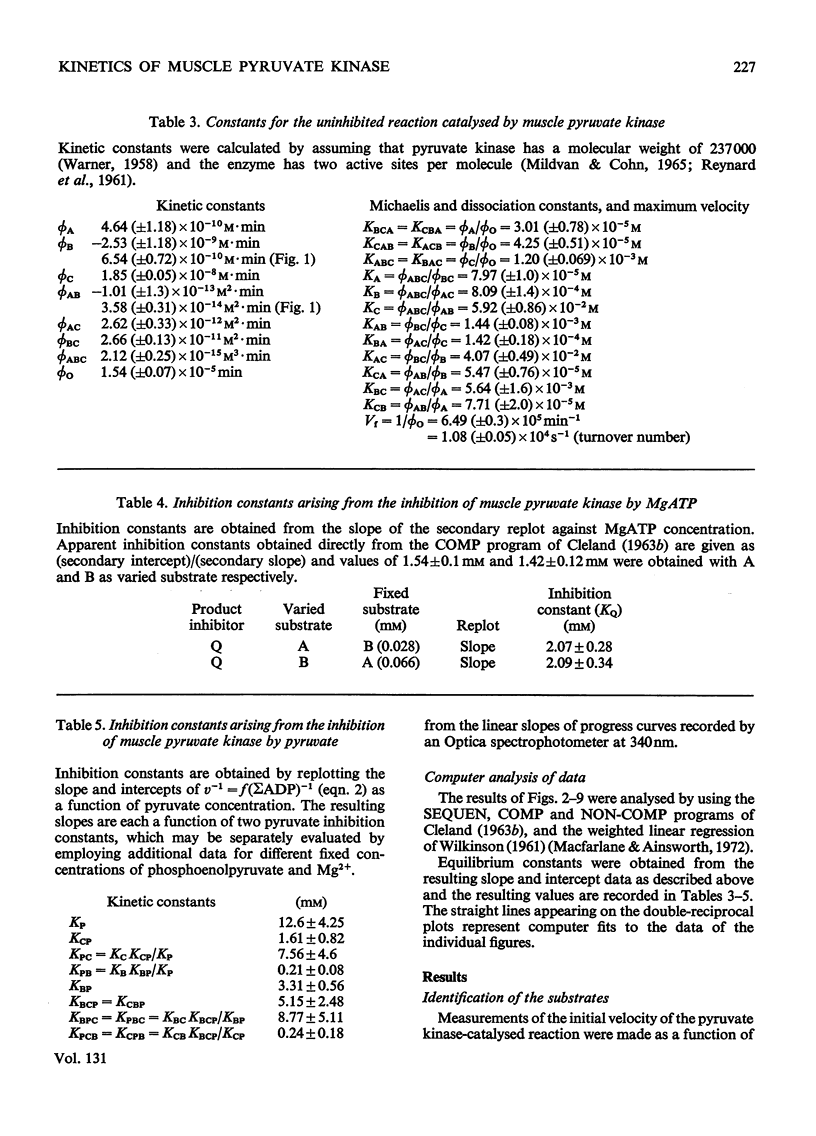
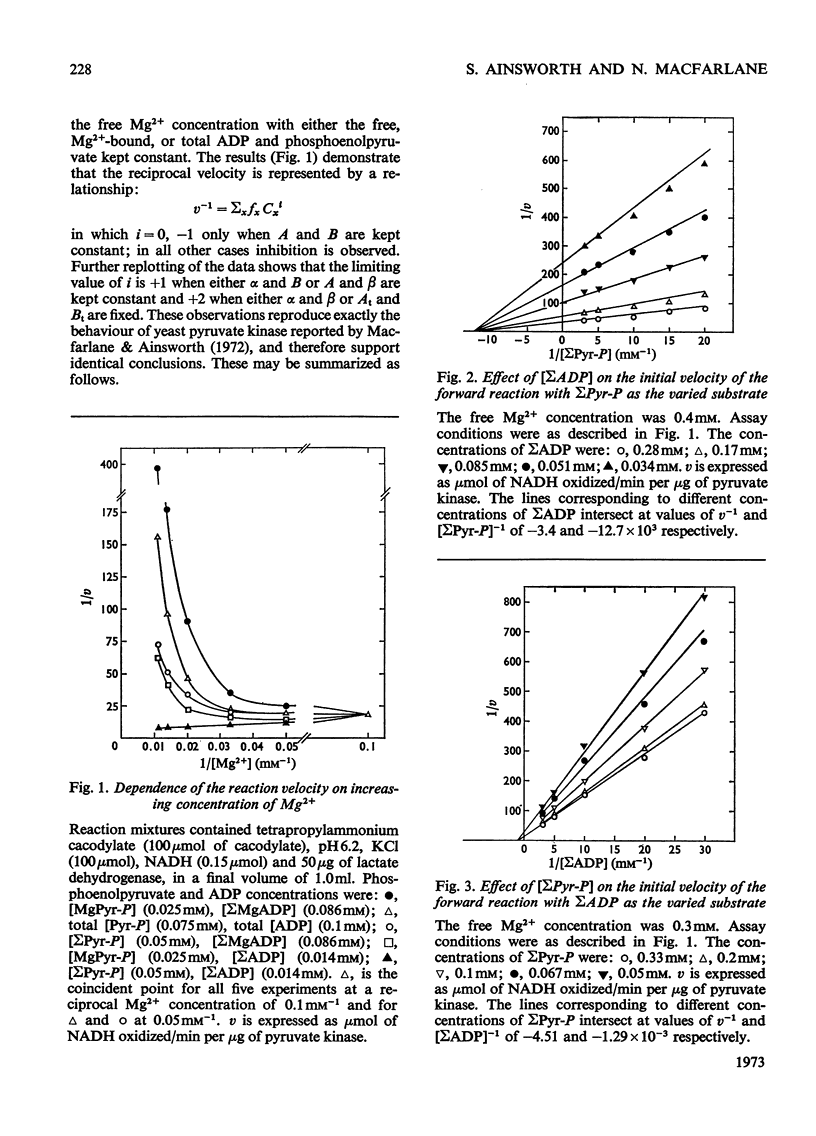
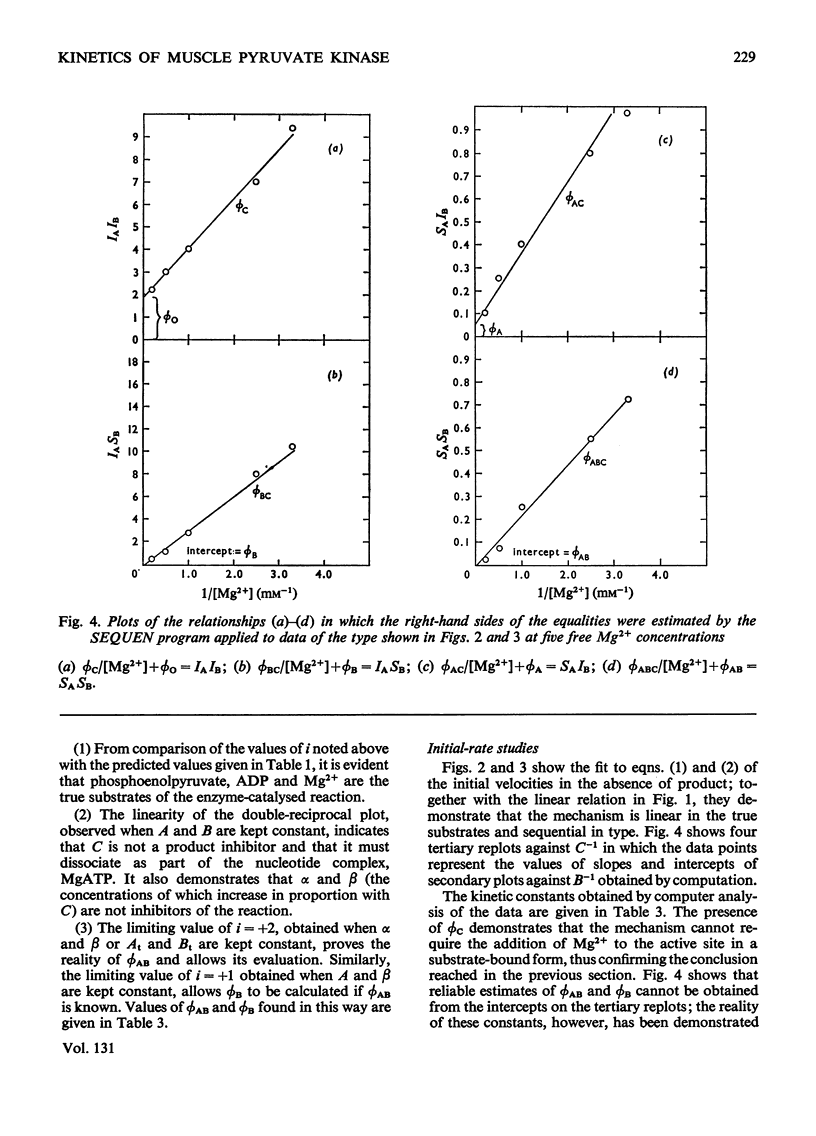
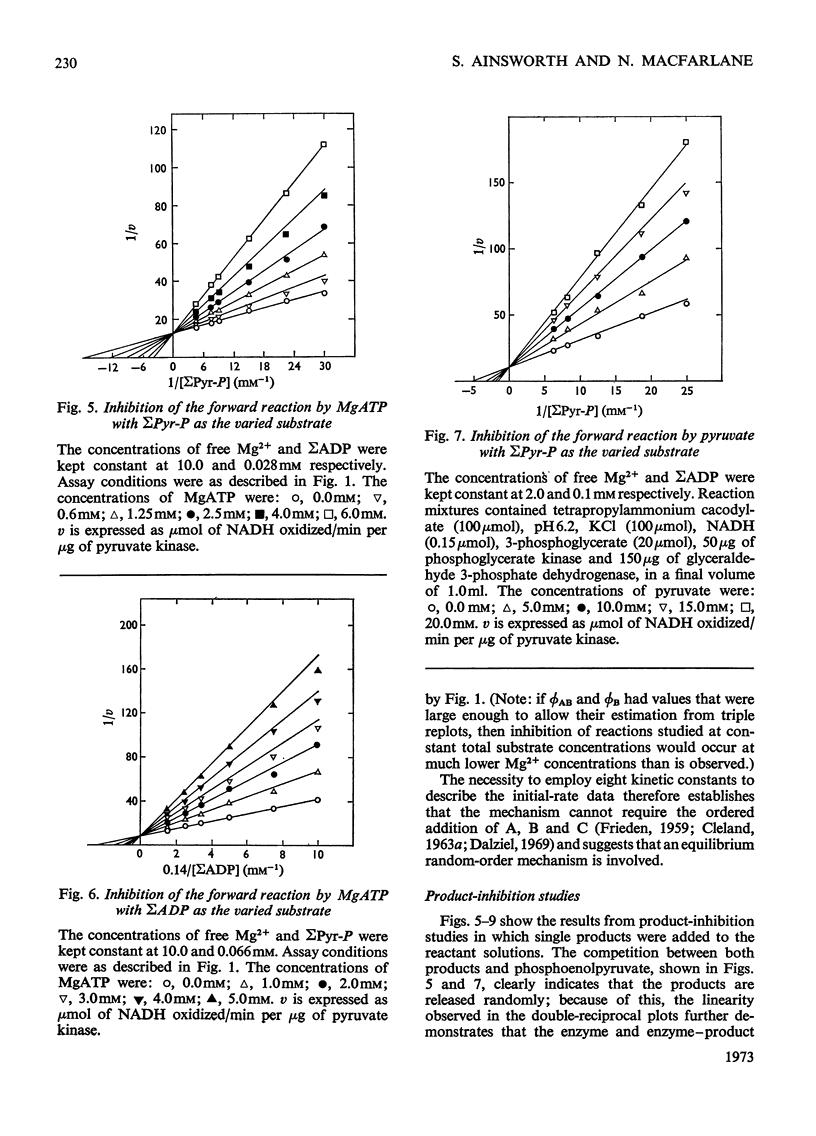
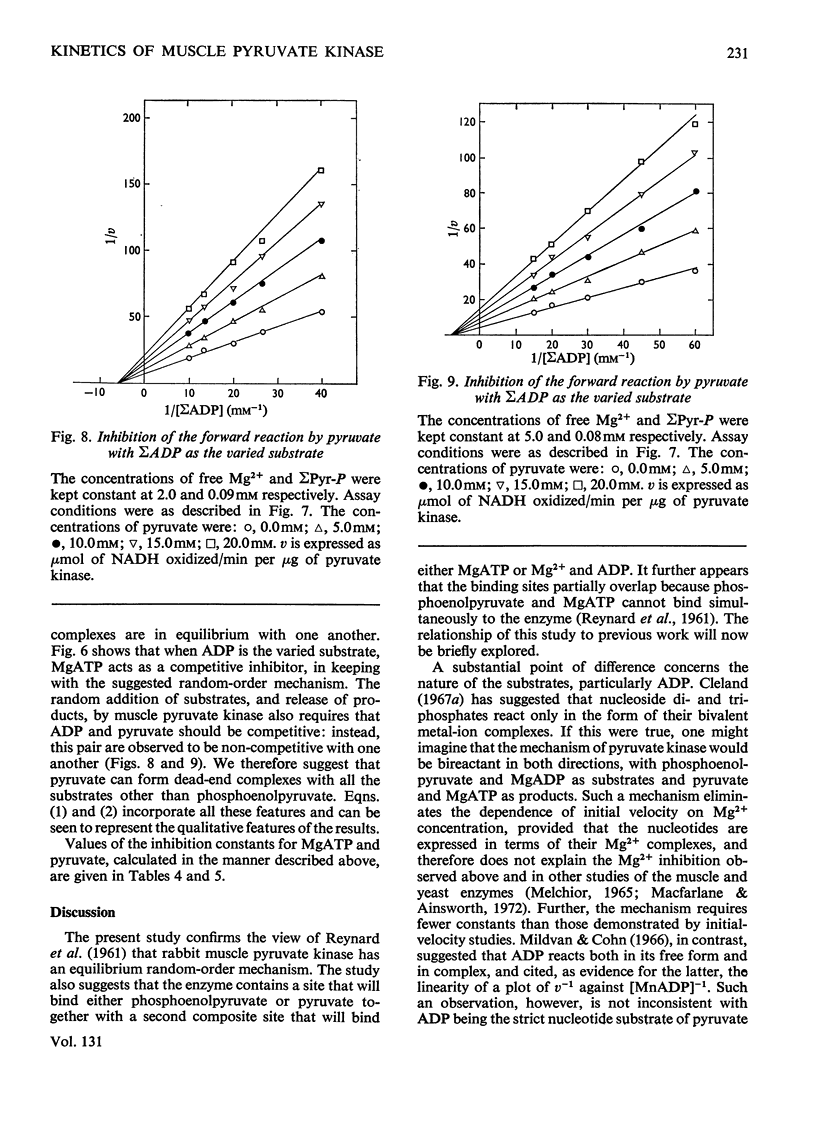
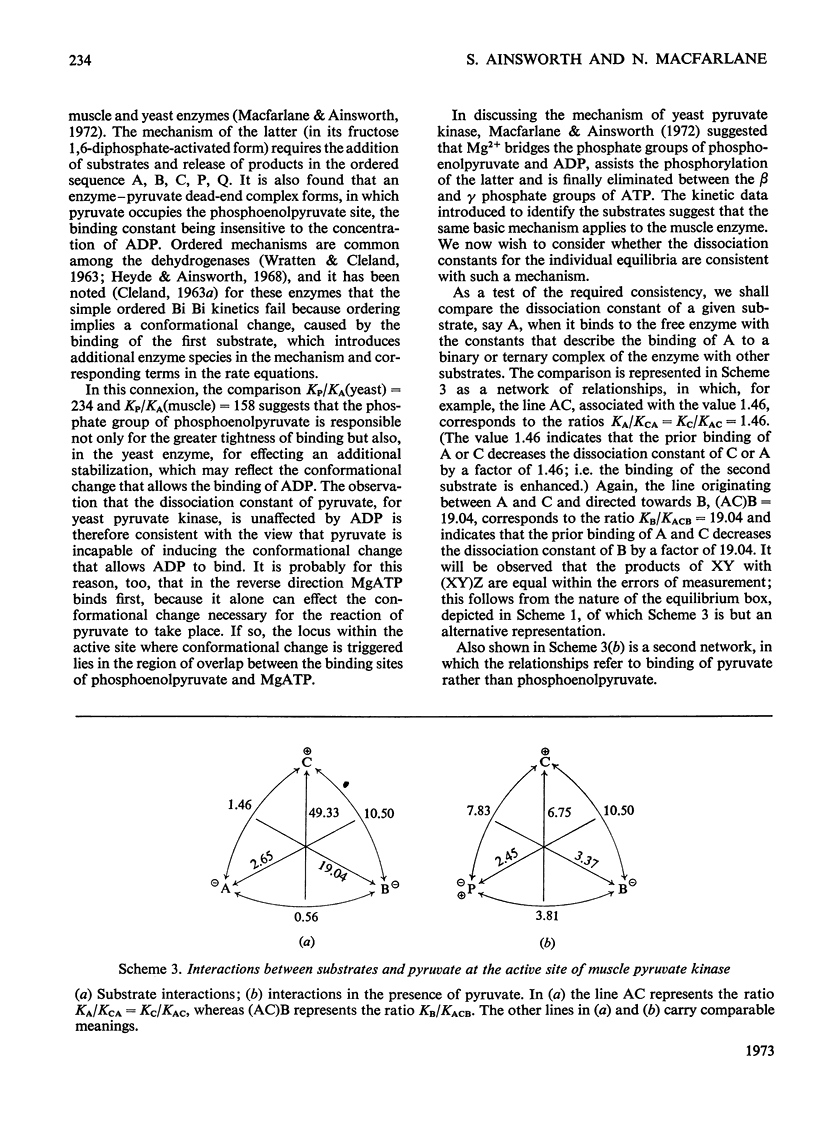
The paper reports a study of the kinetics of the reaction between phosphoenolpyruvate, ADP and Mg2+ catalysed by rabbit muscle pyruvate kinase. The experimental results indicate that the reaction mechanism is equilibrium random-order in type, that the substrates and products are phosphoenolpyruvate, ADP, Mg2+, pyruvate and MgATP, and that dead-end complexes, between pyruvate, ADP and Mg2+, form randomly and exist in equilibrium with themselves and other substrate complexes. Values were determined for the Michaelis, dissociation and inhibition constants of the reaction and are compared with values ascertained by previous workers.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOCK R. M., LING N. S., MORELL S. A., LIPTON S. H. Ultraviolet absorption spectra of adenosine-5'-triphosphate and related 5'-ribonucleotides. Arch Biochem Biophys. 1956 Jun;62(2):253–264. doi: 10.1016/0003-9861(56)90123-0. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. Computer programmes for processing enzyme kinetic data. Nature. 1963 May 4;198:463–465. doi: 10.1038/198463a0. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Dalziel K. The interpretation of kinetic data for enzyme-catalysed reactions involving three substrates. Biochem J. 1969 Sep;114(3):547–556. doi: 10.1042/bj1140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDEN C. Glutamic dehydrogenase. III. The order of substrate addition in the enzymatic reaction. J Biol Chem. 1959 Nov;234:2891–2896. [PubMed] [Google Scholar]
- Heyde E., Ainsworth S. Kinetic studies on the mechanism of the malate dehydrogenase reaction. J Biol Chem. 1968 May 10;243(9):2413–2423. [PubMed] [Google Scholar]
- Hunsley J. R., Suelter C. H. Yeast pyruvate kinase. II. Kinetic properties. J Biol Chem. 1969 Sep 25;244(18):4819–4822. [PubMed] [Google Scholar]
- KACHMAR J. F., BOYER P. D. Kinetic analysis of enzyme reactions. II. The potassium activation and calcium inhibition of pyruvic phosphoferase. J Biol Chem. 1953 Feb;200(2):669–682. [PubMed] [Google Scholar]
- KAYNE F. J., SUELTER C. H. EFFECTS OF TEMPERATURE, SUBSTRATE, AND ACTIVATING CATIONS ON THE CONFORMATIONS OF PYRUVATE KINASE IN AQUEOUS SOLUTIONS. J Am Chem Soc. 1965 Feb 20;87:897–900. doi: 10.1021/ja01082a035. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R. Some reactions of adenosine and inosine phosphates in animal tissues. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):172–180. doi: 10.1016/0006-3002(53)90136-x. [DOI] [PubMed] [Google Scholar]
- Kuczenski R. T., Suelter C. H. Effect of temperature and effectors on the conformations of yeast pyruvate kinase. Biochemistry. 1970 Feb 17;9(4):939–945. doi: 10.1021/bi00806a033. [DOI] [PubMed] [Google Scholar]
- MILDVAN A. S., COHN M. KINETIC AND MAGNETIC RESONANCE STUDIES OF THE PYRUVATE KINASE REACTION. I. DIVALENT METAL COMPLEXES OF PYRUVATE KINASE. J Biol Chem. 1965 Jan;240:238–246. [PubMed] [Google Scholar]
- Macfarlane N., Ainsworth S. A kinetic study of Baker's-yeast pyruvate kinase activated by fructose 1,6-diphosphate. Biochem J. 1972 Oct;129(5):1035–1047. doi: 10.1042/bj1291035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQUATE J. T., UTTER M. F. Equilibrium and kinetic studies of the pyruvic kinase reaction. J Biol Chem. 1959 Aug;234(8):2151–2157. [PubMed] [Google Scholar]
- Melchior J. B. The role of metal ions in the pyruvic kinase reaction. Biochemistry. 1965 Aug;4(8):1518–1525. doi: 10.1021/bi00884a009. [DOI] [PubMed] [Google Scholar]
- Mildvan A. S., Cohn M. Kinetic and magnetic resonance studies of the pyruvate kinase reaction. II. Complexes of enzyme, metal, and substrates. J Biol Chem. 1966 Mar 10;241(5):1178–1193. [PubMed] [Google Scholar]
- PHILLIPS R. C., GEORGE P., RUTMAN R. J. POTENTIOMETRIC STUDIES OF THE SECONDARY PHOSPHATE IONIZATIONS OF AMP, ADP, AND ATP, AND CALCULATIONS OF THERMODYNAMIC DATA FOR THE HYDROLYSIS REACTIONS. Biochemistry. 1963 May-Jun;2:501–508. doi: 10.1021/bi00903a019. [DOI] [PubMed] [Google Scholar]
- Pogson C. I. Adipose-tissue pyruvate kinase. Properties and interconversion of two active forms. Biochem J. 1968 Nov;110(1):67–77. doi: 10.1042/bj1100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon N. G., Bondar R. J. A direct spectrophotometric assay for pyruvate kinase. Anal Biochem. 1967 May;19(2):272–279. doi: 10.1016/0003-2697(67)90163-7. [DOI] [PubMed] [Google Scholar]
- REYNARD A. M., HASS L. F., JACOBSEN D. D., BOYER P. D. The correlation of reaction kinetics and substrate binding with the mechanism of pyruvate kinase. J Biol Chem. 1961 Aug;236:2277–2283. [PubMed] [Google Scholar]
- ROSE I. A. Studies on the enolization of pyruvate by pyruvate kinase. J Biol Chem. 1960 Apr;235:1170–1177. [PubMed] [Google Scholar]
- Rose I. A., O'Connell E. L., Noce P., Utter M. F., Wood H. G., Willard J. M., Cooper T. G., Benziman M. Stereochemistry of the enzymatic carboxylation of phosphoenolpyruvate. J Biol Chem. 1969 Nov 25;244(22):6130–6133. [PubMed] [Google Scholar]
- Rose I. A. Stereochemistry of pyruvate kinase, pyruvate carboxylase, and malate enzyme reactions. J Biol Chem. 1970 Nov 25;245(22):6052–6056. [PubMed] [Google Scholar]
- Siebert G., Kesselring K., Fischer F. Phosphoenolpyruvat als Substrat der alkalischen Phosphatase: Konkurrenz mit Pyruvatkinase. Hoppe Seylers Z Physiol Chem. 1965;341(1):44–75. [PubMed] [Google Scholar]
- WARNER R. C. Physical properties of crystalline fluorokinase. Arch Biochem Biophys. 1958 Dec;78(2):494–496. doi: 10.1016/0003-9861(58)90373-4. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRATTEN C. C., CLELAND W. W. PRODUCT INHIBITION STUDIES ON YEAST AND LIVER ALCOHOL DEHYDROGENASES. Biochemistry. 1963 Sep-Oct;2:935–941. doi: 10.1021/bi00905a007. [DOI] [PubMed] [Google Scholar]



