Abstract
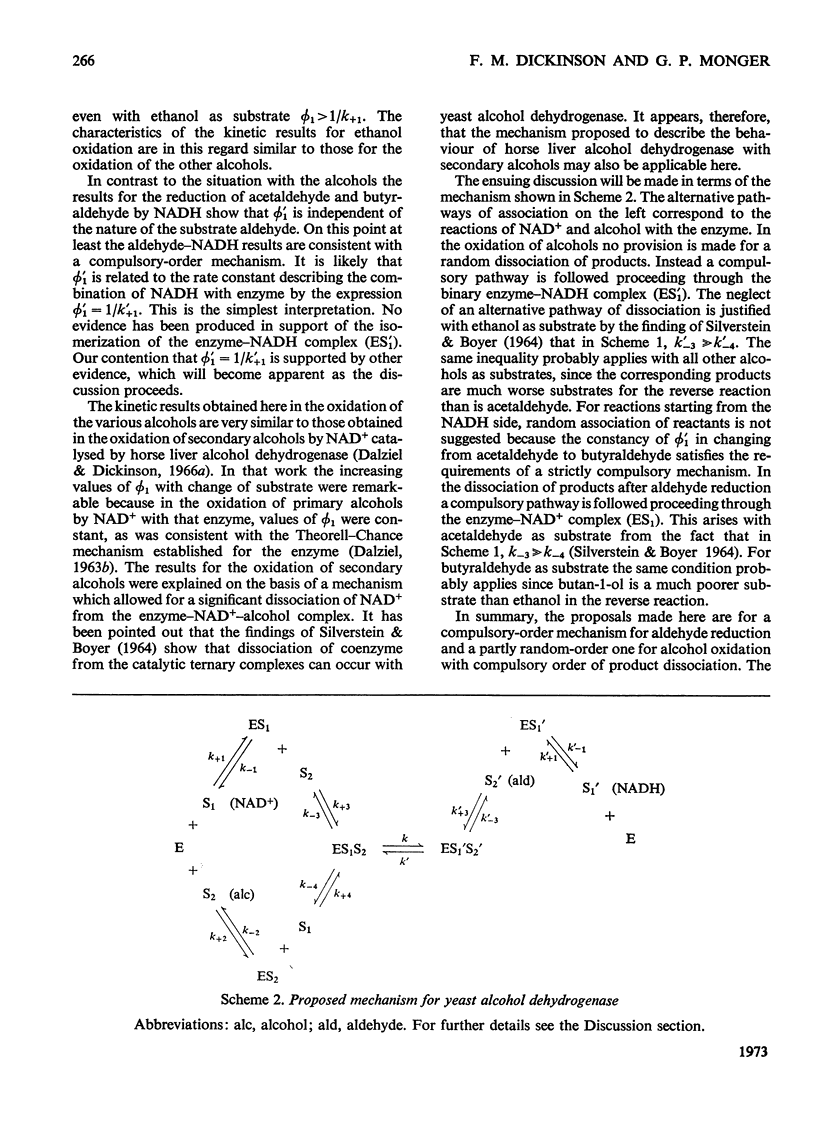
1. The kinetics of oxidation of ethanol, propan-1-ol, butan-1-ol and propan-2-ol by NAD+ and of reduction of acetaldehyde and butyraldehyde by NADH catalysed by yeast alcohol dehydrogenase were studied. 2. Results for the aldehyde–NADH reactions are consistent with a compulsory-order mechanism with the rate-limiting step being the dissociation of the product enzyme–NAD+ complex. In contrast the results for the alcohol–NAD+ reactions indicate that some dissociation of coenzyme from the active enzyme–NAD+–alcohol ternary complexes must occur and that the mechanism is not strictly compulsory-order. The rate-limiting step in ethanol oxidation is the dissociation of the product enzyme–NADH complex but with the other alcohols it is probably the catalytic interconversion of ternary complexes. 3. The rate constants describing the combination of NAD+ and NADH with the enzyme and the dissociations of these coenzymes from binary complexes with the enzyme were measured.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks R. L., Shore J. D. Effect of substrate structure on the rate of the catalytic step in the liver alcohol dehydrogenase mechanism. Biochemistry. 1971 Oct 12;10(21):3855–3858. doi: 10.1021/bi00797a009. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. KINETIC STUDIES OF LIVER ALCOHOL DEHYDROGENASE AND PH EFFECTS WITH COENZYME PREPARATIONS OF HIGH PURITY. J Biol Chem. 1963 Aug;238:2850–2858. [PubMed] [Google Scholar]
- DALZIEL K. Kinetic studies of liver alcohol dehydrogenase. Biochem J. 1962 Aug;84:244–254. doi: 10.1042/bj0840244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. Some observations on the preparation and properties of dihydronicotinamide-adenine dinucleotide. Biochem J. 1962 Aug;84:240–244. doi: 10.1042/bj0840240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. The purification of nicotinamide adenine dinucleotide and kinetic effects of nucleotide impurities. J Biol Chem. 1963 Apr;238:1538–1543. [PubMed] [Google Scholar]
- Dalziel K., Dickinson F. M. Aldehyde mutase. Nature. 1965 Apr 17;206(981):255–257. doi: 10.1038/206255a0. [DOI] [PubMed] [Google Scholar]
- Dalziel K., Dickinson F. M. Substrate activation and inhibition in coenzyme-substrate reactions cyclohexanol oxidation catalysed by liver alcohol dehydrogenase. Biochem J. 1966 Aug;100(2):491–500. doi: 10.1042/bj1000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel K., Dickinson F. M. The kinetics and mechanism of liver alcohol dehydrogenase with primary and secondary alcohols as substrates. Biochem J. 1966 Jul;100(1):34–46. doi: 10.1042/bj1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M. Role of the essential thiol groups of yeast alcohol dehydrogenase. Biochem J. 1972 Jan;126(1):133–138. doi: 10.1042/bj1260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M. The binding of dihydronicotinamide--adenine dinucleotide and pyridine-3-aldehyde--adenine dinucleotide by yeast alcohol dehydrogenase. Biochem J. 1970 Dec;120(4):821–830. doi: 10.1042/bj1200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYES J. E., Jr, VELICK S. F. Yeast alcohol dehydrogenase: molecular weight, coenzyme binding, and reaction equilibria. J Biol Chem. 1954 Mar;207(1):225–244. [PubMed] [Google Scholar]
- MAHLER H. R., BAKER R. H., Jr, SHINER V. J., Jr Studies on the mechanism of enzyme-catalyzed oxidation reduction reactions. IV. A proposed mechanism for the over-all reaction catalyzed by liver alcohol dehydrogenase. Biochemistry. 1962 Jan;1:47–52. doi: 10.1021/bi00907a008. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN E., BOYER P. D. EQUILIBRIUM REACTION RATES AND THE MECHANISMS OF LIVER AND YEAST ALCOHOL DEHYDROGENASE. J Biol Chem. 1964 Nov;239:3908–3914. [PubMed] [Google Scholar]
- WONG J. T., HANES C. S. ISOTOPIC EXCHANGE AT EQUILIBRIUM AS A CRITERION OF ENZYMATIC MECHANISMS. Nature. 1964 Aug 1;203:492–494. doi: 10.1038/203492a0. [DOI] [PubMed] [Google Scholar]
- WRATTEN C. C., CLELAND W. W. PRODUCT INHIBITION STUDIES ON YEAST AND LIVER ALCOHOL DEHYDROGENASES. Biochemistry. 1963 Sep-Oct;2:935–941. doi: 10.1021/bi00905a007. [DOI] [PubMed] [Google Scholar]


