Abstract
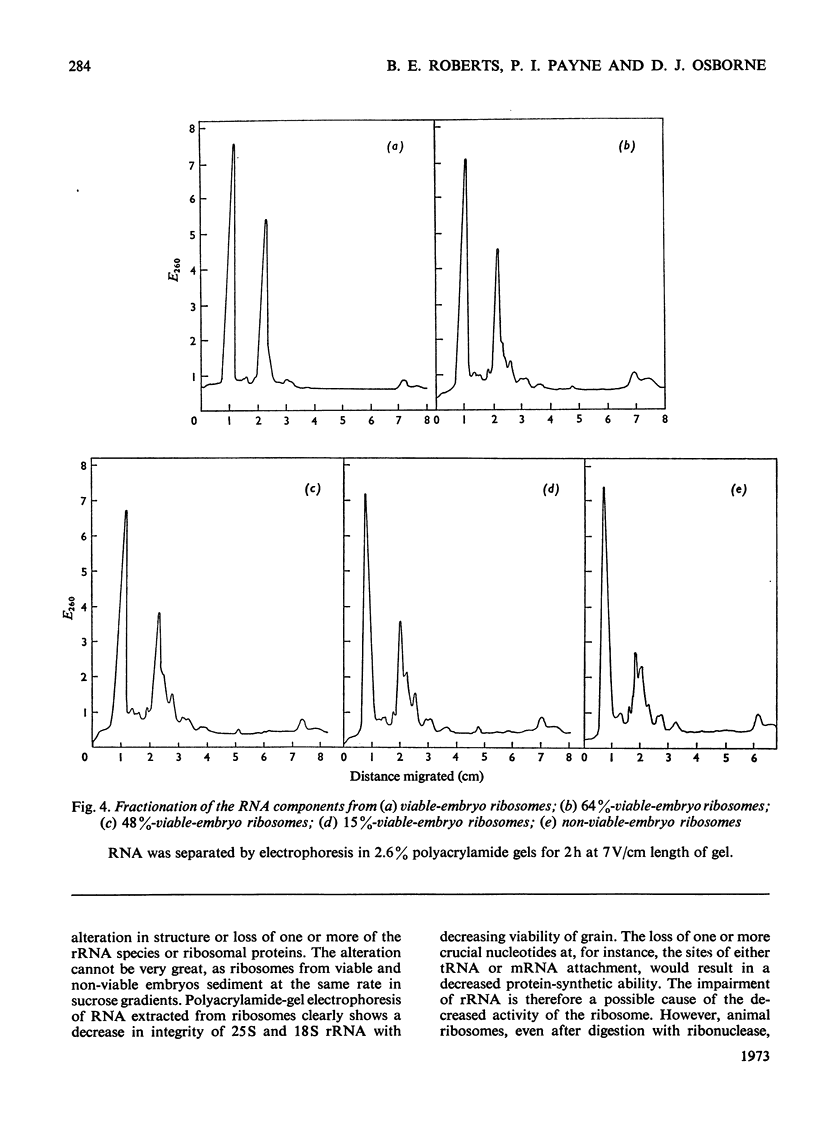
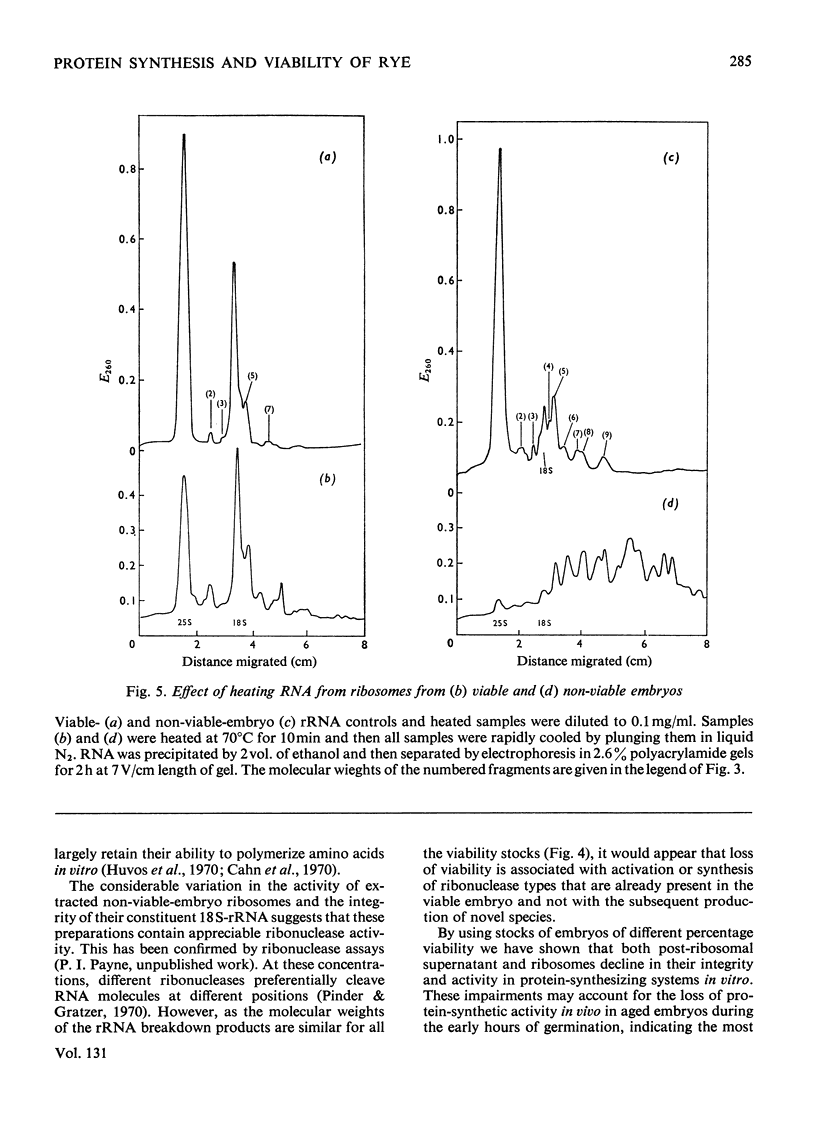
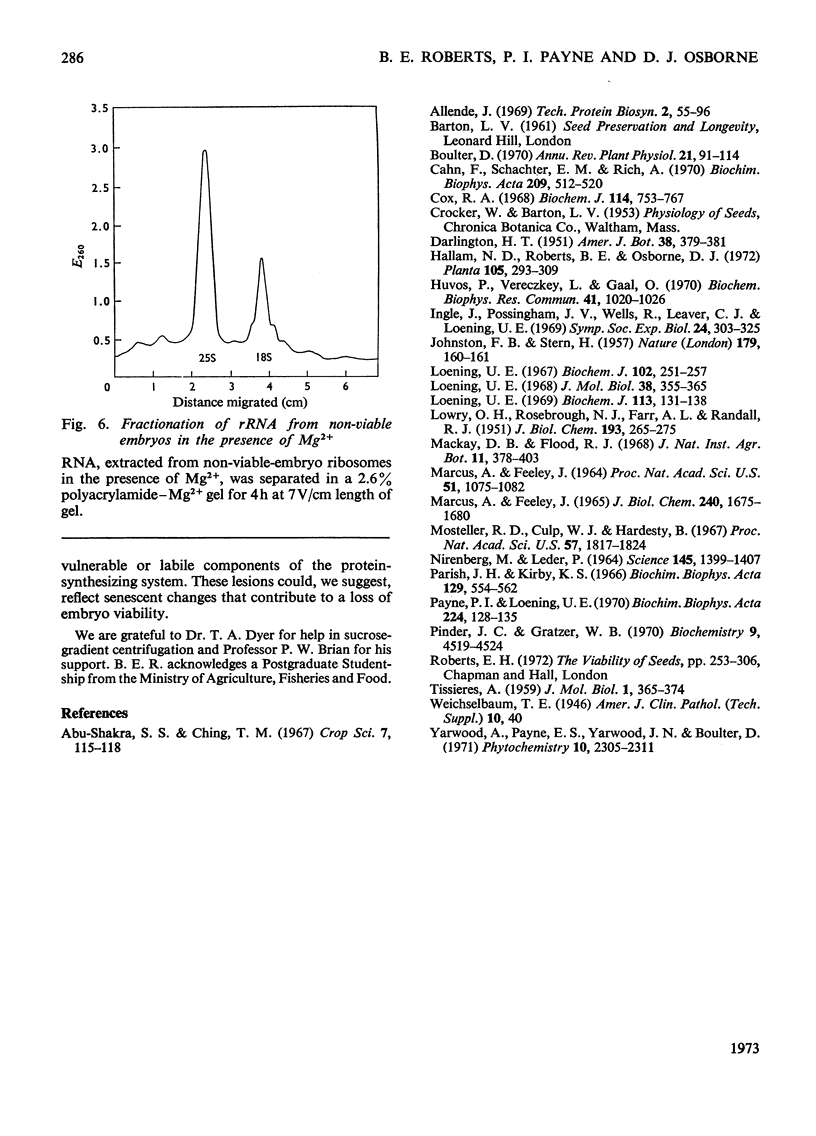
A study was made of the integrity of some components of the protein-synthesizing system from viable and non-viable embryos of rye grains. In comparison with viable-embryo components both post-ribosomal supernatant and ribosomal fractions from non-viable embryos are impaired, for neither will fully support polyphenylalanine synthesis in poly(U)-directed cell-free systems. The lesion in the supernatant lies in components other than the tRNA or the aminoacyl-tRNA synthetase, for these are as functional as those present in the fully active cell-free systems from viable embryos. The ribosomes of embryos of lowered viability show considerable fragmentation and degradation of both 18S and 25S rRNA. This breakdown does not, however, account for the complete lack of polypeptide synthesis in the poly(U)-directed non-viable-embryo system, for if provided with viable-embryo supernatant, non-viable-embryo ribosomes will sustain 60% of the viable-embryo ribosome activity. A lesion in non-viable-embryo supernatant has been located in the binding of the aminoacyl-tRNA to the ribosome. The impaired components in both supernatant and ribosomes in systems in vitro may reflect the site of faults in protein synthesis in vivo in the early hours of germination. The development of these lesions during grain storage could contribute to senescence and loss of viability in the embryos of rye.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cahn F., Schachter E. M., Rich A. Polypeptide synthesis with ribonuclease-digested ribosomes. Biochim Biophys Acta. 1970;209(2):512–520. doi: 10.1016/0005-2787(70)90748-3. [DOI] [PubMed] [Google Scholar]
- Cox R. A. The effect of pancreatic ribonuclease on rabbit reticulocyte ribosomes and its interpretation in terms of ribosome structure. Biochem J. 1969 Oct;114(4):753–767. doi: 10.1042/bj1140753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüvös P., Vereczkey L., Gaál O. Incorporating activity of ribosomes and integrity of ribosomal RNA. Biochem Biophys Res Commun. 1970 Nov 25;41(4):1020–1026. doi: 10.1016/0006-291x(70)90187-7. [DOI] [PubMed] [Google Scholar]
- Ingle J., Possingham J. V., Wells R., Leaver C. J., Loening U. E. The properties of chloroplast ribosomal-RNA. Symp Soc Exp Biol. 1970;24:303–325. [PubMed] [Google Scholar]
- JOHNSTON F. B., STERN H. Mass isolation of viable wheat embryos. Nature. 1957 Jan 19;179(4551):160–161. doi: 10.1038/179160b0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCUS A., FEELEY J. PROTEIN SYNTHESIS IN IMBIBED SEEDS. II. POLYSOME FORMATION DURING IMBIBITION. J Biol Chem. 1965 Apr;240:1675–1680. [PubMed] [Google Scholar]
- Marcus A., Feeley J. ACTIVATION OF PROTEIN SYNTHESIS IN THE IMBIBITION PHASE OF SEED GERMINATION. Proc Natl Acad Sci U S A. 1964 Jun;51(6):1075–1079. doi: 10.1073/pnas.51.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteller R. D., Culp W. J., Hardesty B. A reaction associated with nonenzymatic binding in the reticulocyte transfer system. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1817–1824. doi: 10.1073/pnas.57.6.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Parish J. H., Kirby K. S. Reagents which reduce interactions between ribosomal RNA and rapidly labelled RNA from rat liver. Biochim Biophys Acta. 1966 Dec 21;129(3):554–562. doi: 10.1016/0005-2787(66)90070-0. [DOI] [PubMed] [Google Scholar]
- Payne P. I., Loening U. E. RNA breakdown accompanying the isolation of pea root microsomes. An analysis by polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1970 Nov 12;224(1):128–135. doi: 10.1016/0005-2787(70)90626-x. [DOI] [PubMed] [Google Scholar]
- Pinder J. C., Gratzer W. B. On the basis of specific fragmentation of ribonucleic acid by nucleases. Biochemistry. 1970 Nov 10;9(23):4519–4524. doi: 10.1021/bi00825a010. [DOI] [PubMed] [Google Scholar]


