Abstract
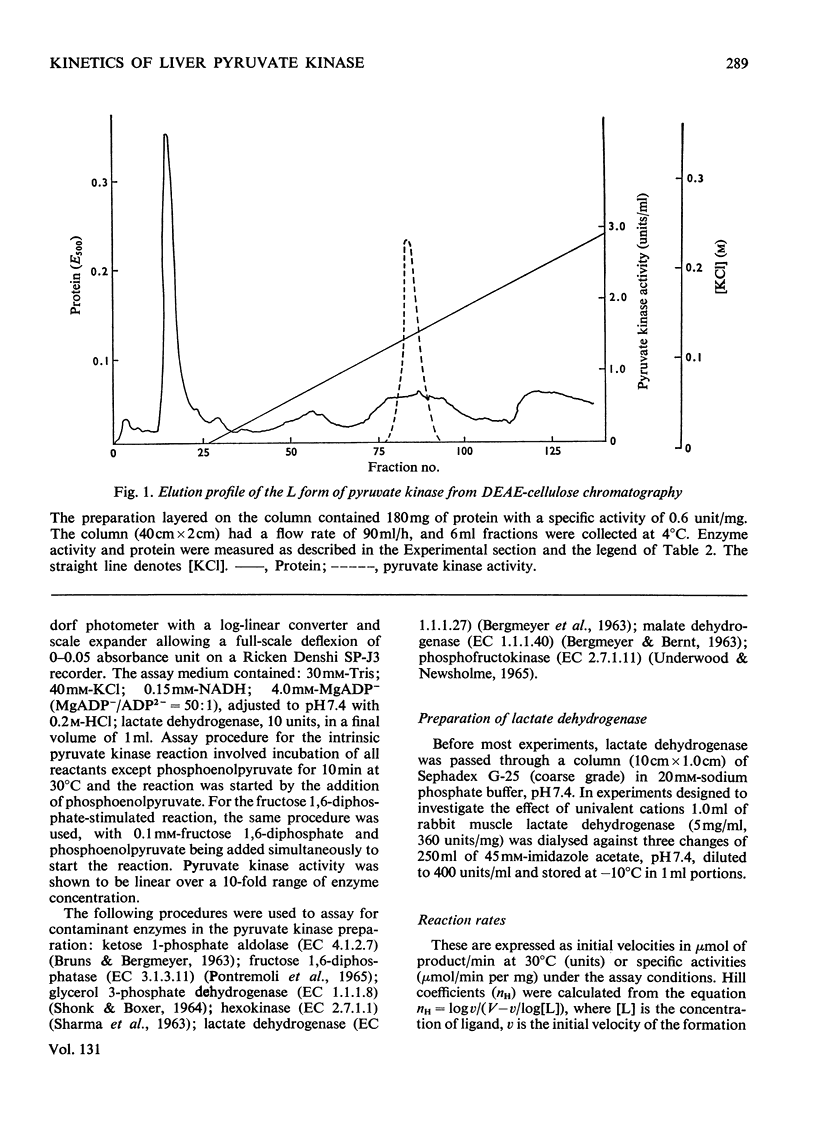
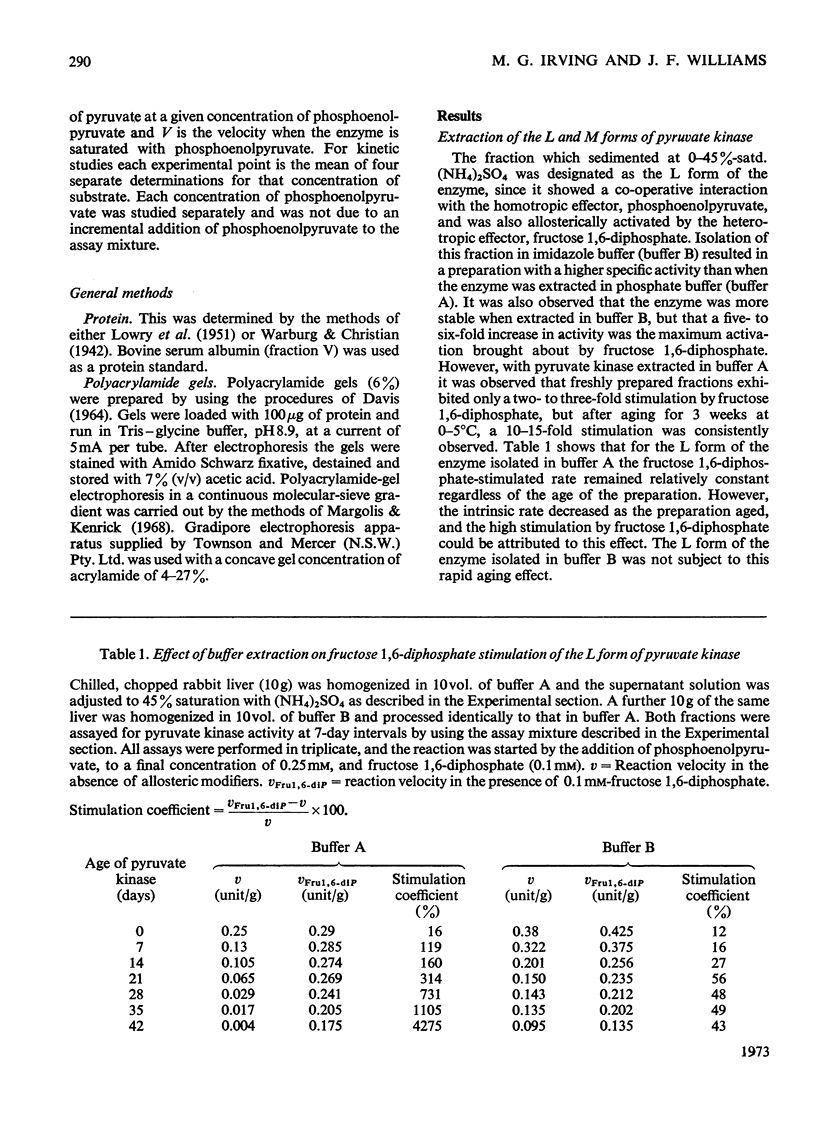
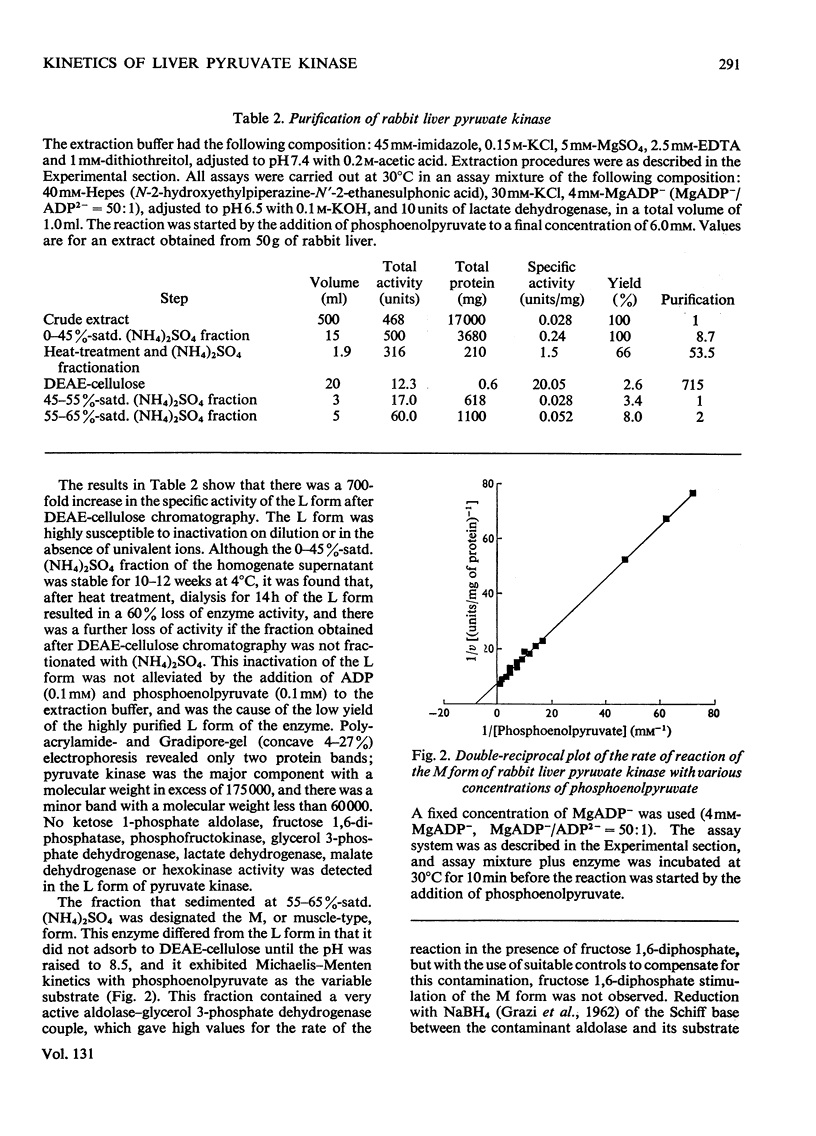
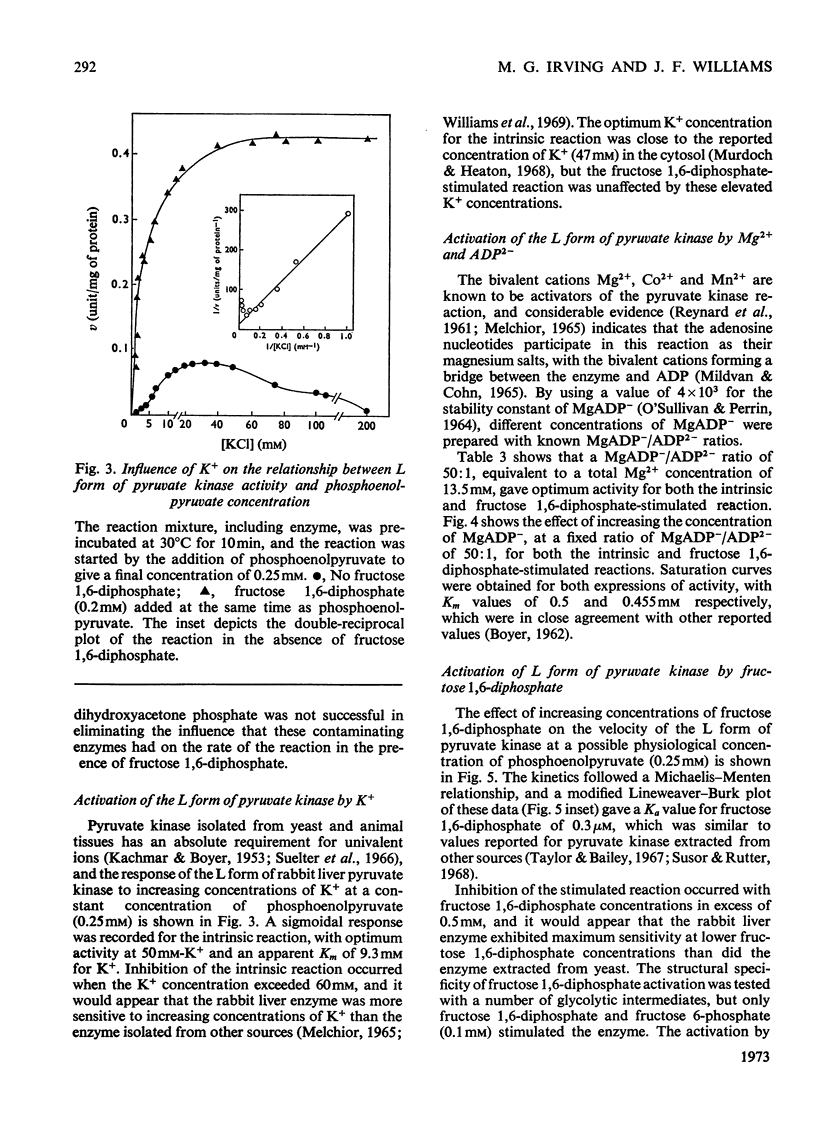
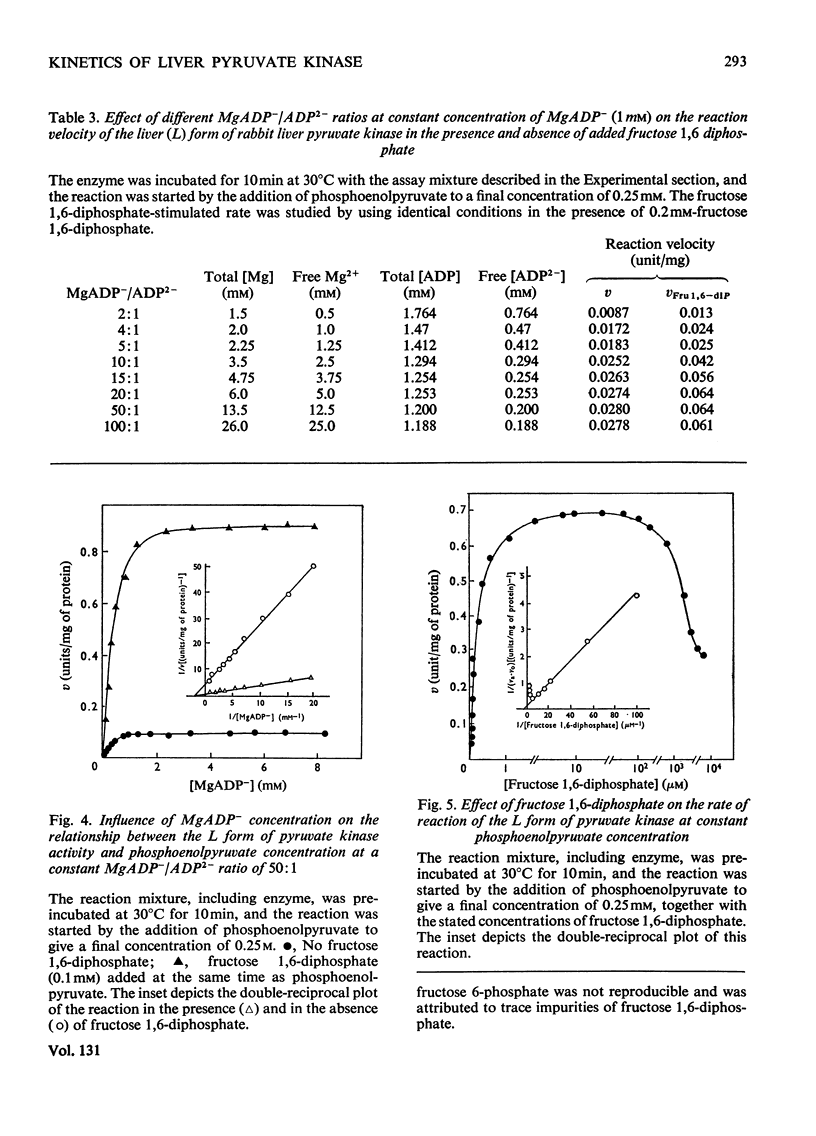
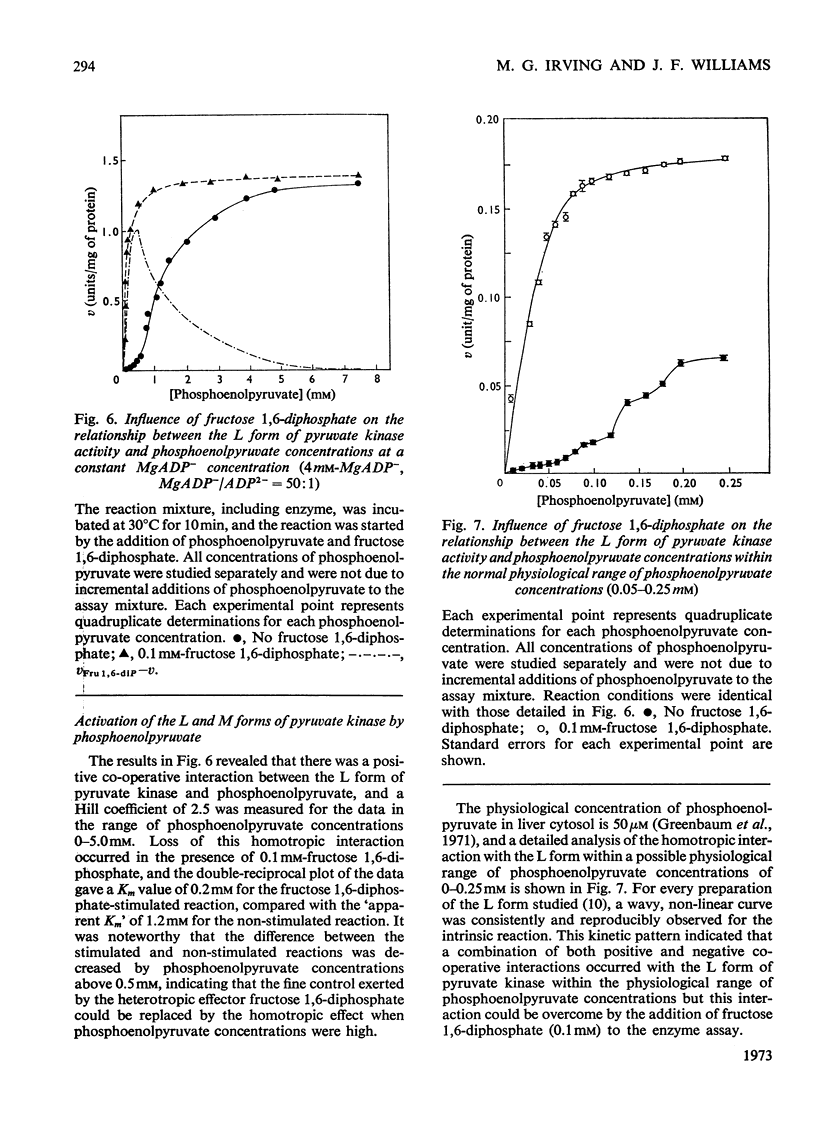
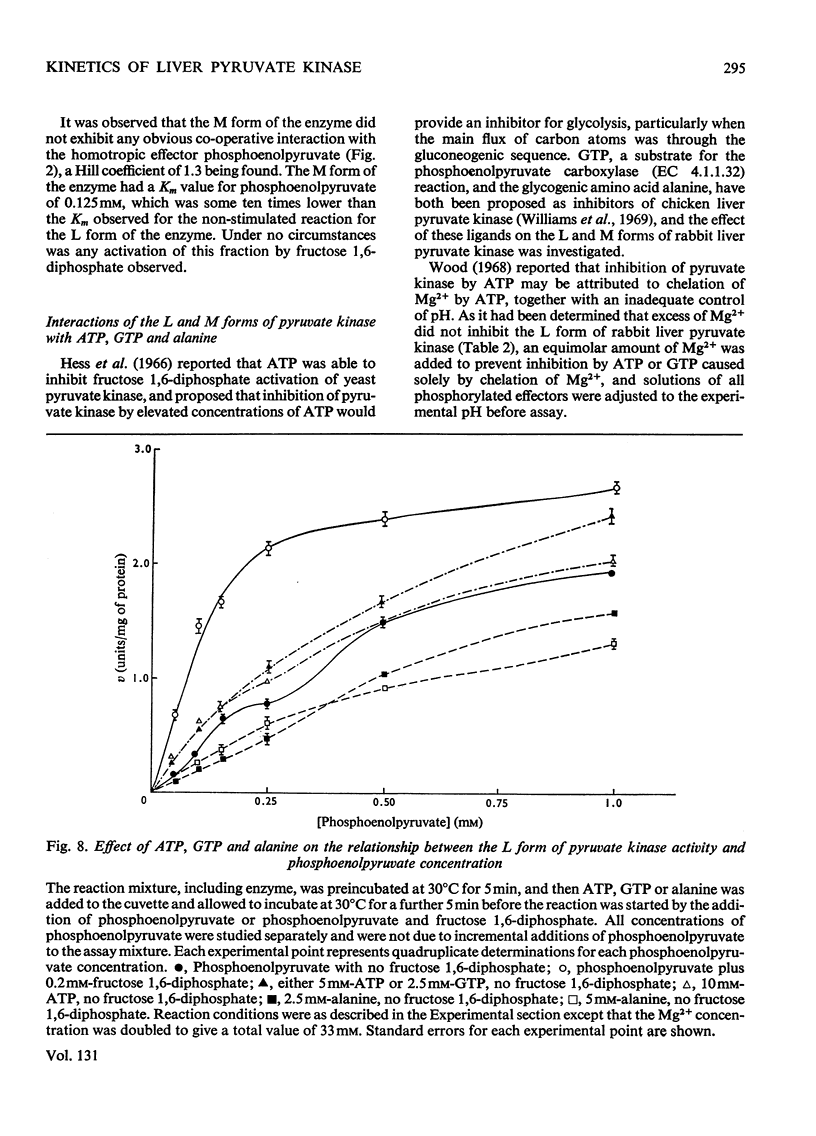
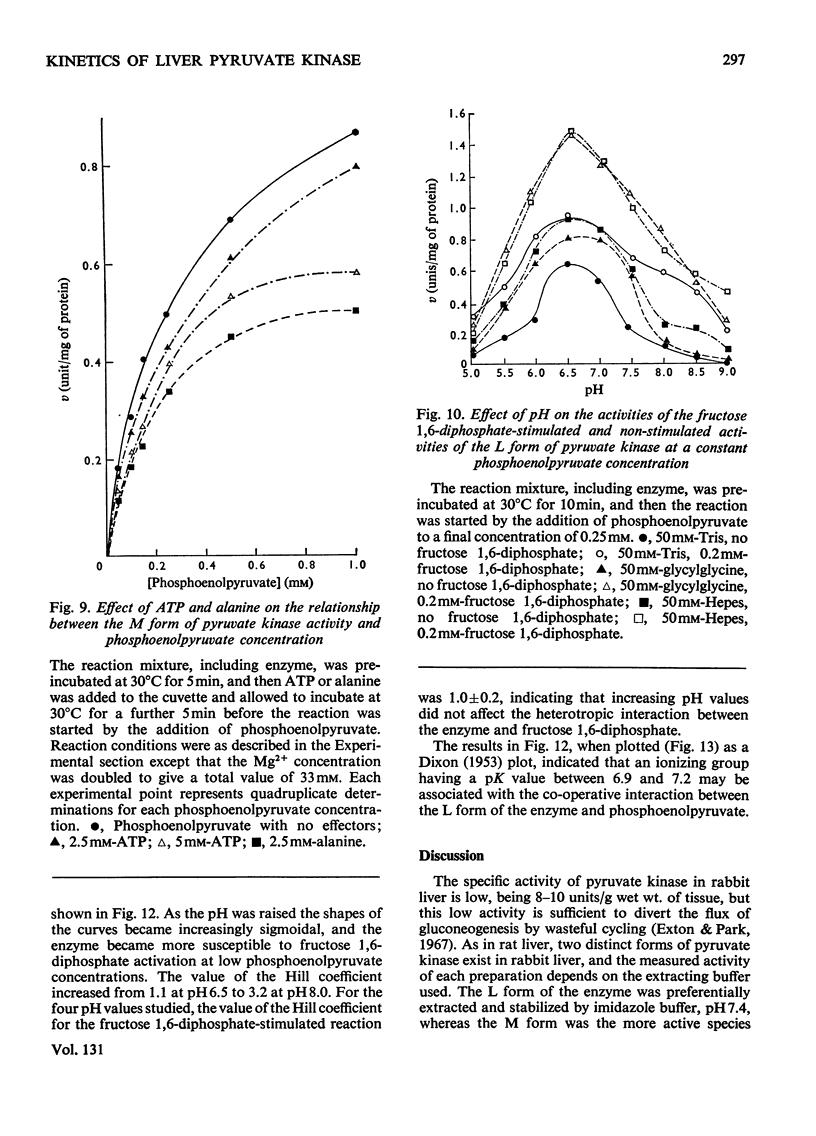
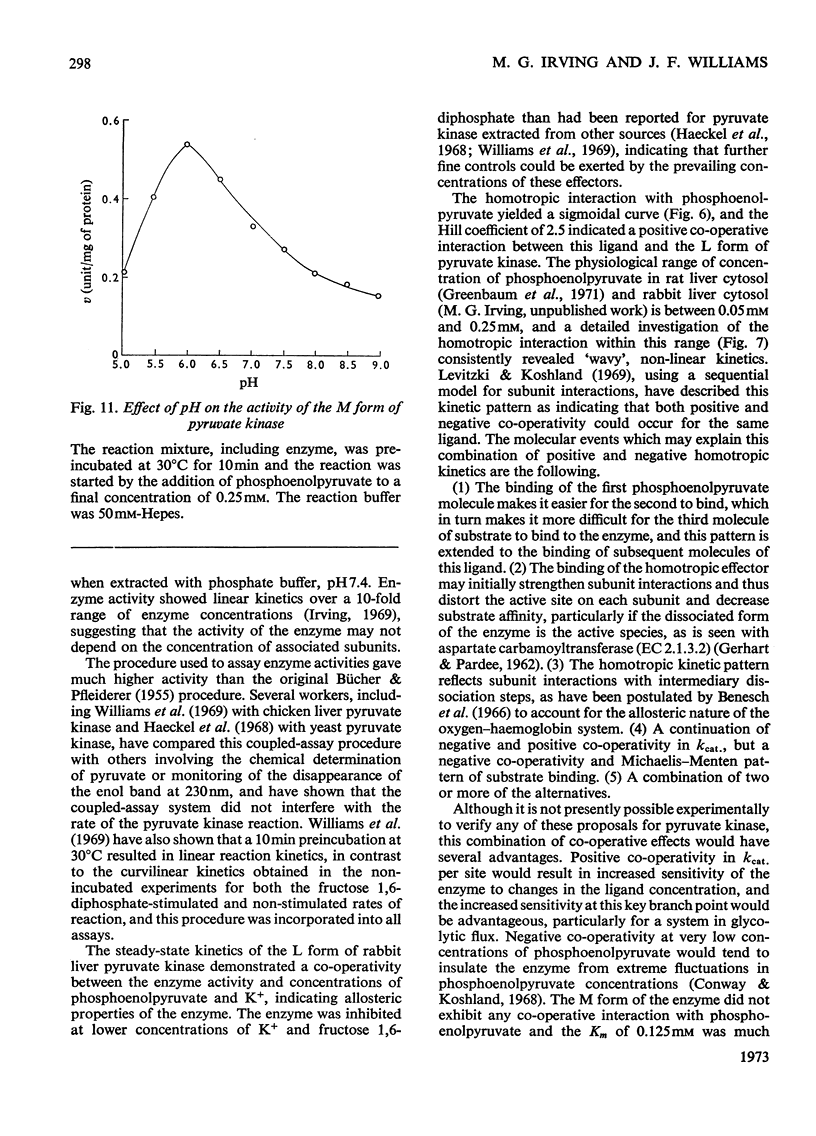
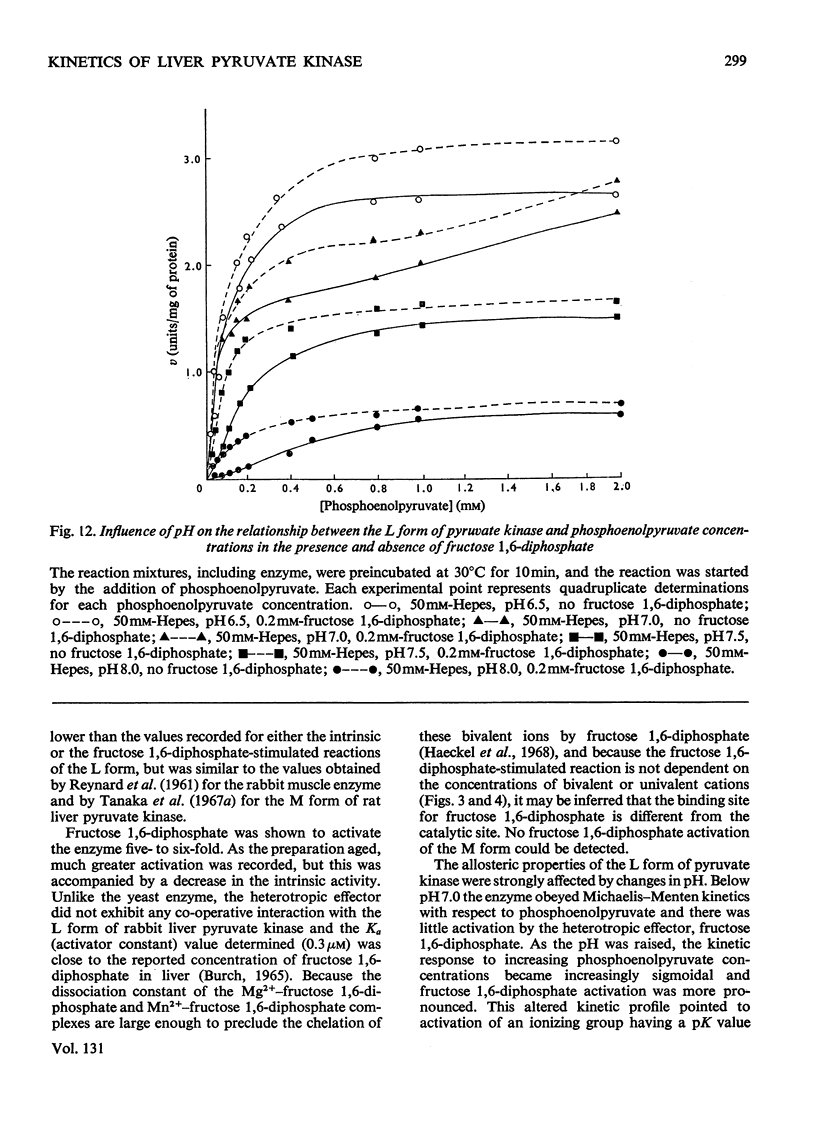
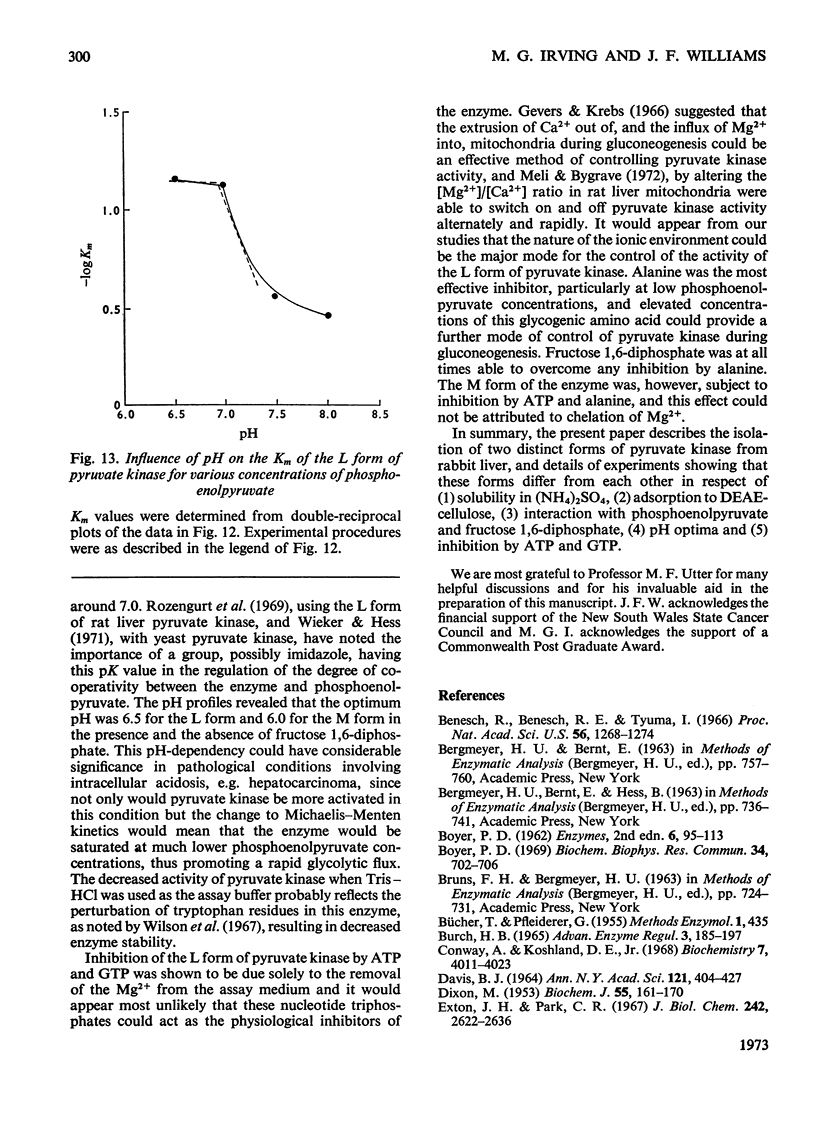
Two kinetically distinct forms of pyruvate kinase (EC 2.7.1.40) were isolated from rabbit liver by using differential ammonium sulphate fractionation. The L or liver form, which is allosterically activated by fructose 1,6-diphosphate, was partially purified by DEAE-cellulose chromatography to give a maximum specific activity of 20 units/mg. The L form was allosterically activated by K+ and optimum activity was recorded with 30mm-K+, 4mm-MgADP−, with a MgADP−/ADP2− ratio of 50:1, but inhibition occurred with K+ concentrations in excess of 60mm. No inhibition occurred with either ATP or GTP when excess of Mg2+ was added to counteract chelation by these ligands. Alanine (2.5mm) caused 50% inhibition at low concentrations of phosphoenolpyruvate (0.15mm). The homotropic effector, phosphoenolpyruvate, exhibited a complex allosteric pattern (nH=2.5), and negative co-operative interactions were observed in the presence of low concentrations of this substrate. The degree of this co-operative interaction was pH-dependent, with the Hill coefficient increasing from 1.1 to 3.2 as the pH was raised from 6.5 to 8.0. Fructose 1,6-diphosphate interfered with the activation by univalent ions, markedly decreased the apparent Km for phosphoenolpyruvate from 1.2mm to 0.2mm, and transformed the phosphoenolpyruvate saturation curve into a hyperbola. Concentrations of fructose 1,6-diphosphate in excess of 0.5mm inhibited this stimulated reaction. The M or muscle-type form of the enzyme was not activated by fructose 1,6-diphosphate and gave a maximum specific activity of 0.3 unit/mg. A Michaelis–Menten response was obtained when phosphoenolpyruvate was the variable substrate (Km=0.125mm), and this form was inhibited by ATP, as well as alanine, even in the presence of excess of Mg2+.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benesch R., Benesch R. E., Tyuma I. Subunit exchange and ligand binding. II. The mechanism of the allosteric effect in hemoglobin. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1268–1274. doi: 10.1073/pnas.56.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P. D. The inhibition of pyruvate kinase by ATP: a Mg++ buffer system for use in enzyme studies. Biochem Biophys Res Commun. 1969 Mar 10;34(5):702–706. doi: 10.1016/0006-291x(69)90795-5. [DOI] [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DIXON M. The effect of pH on the affinities of enzymes for substrates and inhibitors. Biochem J. 1953 Aug;55(1):161–170. doi: 10.1042/bj0550161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- GRAZI E., CHENG T., HORECKER B. L. The formation of a stable aldolase-dihydroxyacetone phosphate complex. Biochem Biophys Res Commun. 1962 Apr 20;7:250–253. doi: 10.1016/0006-291x(62)90184-5. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 9. Effects of fatty acids and ketone bodies, and of alloxan-diabetes and starvation, on pyruvate metabolism and on lactate-pyruvate and L-glycerol 3-phosphate-dihydroxyacetone phosphate concentration ratios in rat heart and rat diaphragm muscles. Biochem J. 1964 Dec;93(3):665–678. doi: 10.1042/bj0930665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers W., Krebs H. A. The effects of adenine nucleotides on carbohydrate metabolism in pigeon-liver homogenates. Biochem J. 1966 Mar;98(3):720–735. doi: 10.1042/bj0980720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A. L., Gumaa K. A., McLean P. The distribution of hepatic metabolites and the control of the pathways of carbohydrate metabolism in animals of different dietary and hormonal status. Arch Biochem Biophys. 1971 Apr;143(2):617–663. doi: 10.1016/0003-9861(71)90247-5. [DOI] [PubMed] [Google Scholar]
- HOMMES F. A. OSCILLATORY REDUCTIONS OF PYRIDINE NUCLEOTIDES DURING ANAEROBIC GLYCOLYSIS IN BREWERS' YEAST. Arch Biochem Biophys. 1964 Oct;108:36–46. doi: 10.1016/0003-9861(64)90352-2. [DOI] [PubMed] [Google Scholar]
- Haeckel R., Hess B., Lauterborn W., Wüster K. H. Purification and allosteric properties of yeast pyruvate kinase. Hoppe Seylers Z Physiol Chem. 1968 May;349(5):699–714. doi: 10.1515/bchm2.1968.349.1.699. [DOI] [PubMed] [Google Scholar]
- Hess B., Haeckel R., Brand K. FDP-activation of yeast pyruvate kinase. Biochem Biophys Res Commun. 1966 Sep 22;24(6):824–831. doi: 10.1016/0006-291x(66)90322-6. [DOI] [PubMed] [Google Scholar]
- Irving M. G., Williams J. F. Studies on the interaction between rabbit liver pyruvate kinase and its allosteric effector fructose 1,6-diphosphate. Biochem J. 1973 Feb;131(2):303–313. doi: 10.1042/bj1310303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KACHMAR J. F., BOYER P. D. Kinetic analysis of enzyme reactions. II. The potassium activation and calcium inhibition of pyruvic phosphoferase. J Biol Chem. 1953 Feb;200(2):669–682. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Negative cooperativity in regulatory enzymes. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1121–1128. doi: 10.1073/pnas.62.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILDVAN A. S., COHN M. KINETIC AND MAGNETIC RESONANCE STUDIES OF THE PYRUVATE KINASE REACTION. I. DIVALENT METAL COMPLEXES OF PYRUVATE KINASE. J Biol Chem. 1965 Jan;240:238–246. [PubMed] [Google Scholar]
- Malcovati M., Kornberg H. L. Two types of pyruvate kinase in Escherichia coli K12. Biochim Biophys Acta. 1969 Apr 22;178(2):420–423. doi: 10.1016/0005-2744(69)90417-3. [DOI] [PubMed] [Google Scholar]
- Margolis J., Kenrick K. G. Polyacrylamide gel electrophoresis in a continuous molecular sieve gradient. Anal Biochem. 1968 Oct 24;25(1):347–362. doi: 10.1016/0003-2697(68)90109-7. [DOI] [PubMed] [Google Scholar]
- Melchior J. B. The role of metal ions in the pyruvic kinase reaction. Biochemistry. 1965 Aug;4(8):1518–1525. doi: 10.1021/bi00884a009. [DOI] [PubMed] [Google Scholar]
- Meli J., Bygrave F. L. The role of mitochondria in modifying calcium-sensitive cytoplasmic metabolic activities. Modification of pyruvate kinase activity. Biochem J. 1972 Jun;128(2):415–420. doi: 10.1042/bj1280415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch J. A., Heaton F. W. Subcellular distribution of metals (sodium, potassium, calcium and magnesium in rat liver, kidney and intestinal mucosa. Comp Biochem Physiol. 1968 Jul;26(1):121–128. doi: 10.1016/0010-406x(68)90318-6. [DOI] [PubMed] [Google Scholar]
- O'SULLIVAN W. J., PERRIN D. D. THE STABILITY CONSTANTS OF METAL-ADENINE NUCLEOTIDE COMPLEXES. Biochemistry. 1964 Jan;3:18–26. doi: 10.1021/bi00889a005. [DOI] [PubMed] [Google Scholar]
- Pogson C. I. Adipose-tissue pyruvate kinase. Properties and interconversion of two active forms. Biochem J. 1968 Nov;110(1):67–77. doi: 10.1042/bj1100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Traniello S., Luppis B., Wood W. A. Fructose diphosphatase from rabbit liver. I. Purification and properties. J Biol Chem. 1965 Sep;240(9):3459–3463. [PubMed] [Google Scholar]
- REYNARD A. M., HASS L. F., JACOBSEN D. D., BOYER P. D. The correlation of reaction kinetics and substrate binding with the mechanism of pyruvate kinase. J Biol Chem. 1961 Aug;236:2277–2283. [PubMed] [Google Scholar]
- Rozengurt E., Jiménez de Asúa L., Carminatti H. Some kinetic properties of liver pyruvate kinase (type L). II. Effect of pH on its allosteric behavior. J Biol Chem. 1969 Jun 25;244(12):3142–3147. [PubMed] [Google Scholar]
- SHARMA C., MANJESHWAR R., WEINHOUSE S. EFFECTS OF DIET AND INSULIN ON GLUCOSE-ADENOSINE TRIPHOSPHATE PHOSPHOTRANSFERASES OF RAT LIVER. J Biol Chem. 1963 Dec;238:3840–3845. [PubMed] [Google Scholar]
- SHONK C. E., BOXER G. E. ENZYME PATTERNS IN HUMAN TISSUES. I. METHODS FOR THE DETERMINATION OF GLYCOLYTIC ENZYMES. Cancer Res. 1964 May;24:709–721. [PubMed] [Google Scholar]
- Suelter C. H., Singleton R., Jr, Kayne F. J., Arrington S., Glass J., Mildvan A. S. Stuies on the interaction of substrate and monovalent and divalent cations with pyruvate kinase. Biochemistry. 1966 Jan;5(1):131–139. doi: 10.1021/bi00865a017. [DOI] [PubMed] [Google Scholar]
- Susor W. A., Rutter W. J. Some distinctive properties of pyruvate kinase purified from rat liver. Biochem Biophys Res Commun. 1968 Jan 11;30(1):14–20. doi: 10.1016/0006-291x(68)90705-5. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Harano Y., Sue F., Morimura H. Crystallization, characterization and metabolic regulation of two types of pyruvate kinase isolated from rat tissues. J Biochem. 1967 Jul;62(1):71–91. doi: 10.1093/oxfordjournals.jbchem.a128639. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Sue F., Morimura H. Feed-forward activation and feed-back inhibition of pyruvate kinase type L of rat liver. Biochem Biophys Res Commun. 1967 Nov 17;29(3):444–449. doi: 10.1016/0006-291x(67)90477-9. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. PROPERTIES OF PHOSPHOFRUCTOKINASE FROM RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLYCOLYSIS AND GLUCONEOGENESIS. Biochem J. 1965 Jun;95:868–875. doi: 10.1042/bj0950868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieker H. J., Hess B. Allosteric interactions of yeast pyruvate kinase as a function of pH. Biochemistry. 1971 Mar 30;10(7):1243–1248. doi: 10.1021/bi00783a022. [DOI] [PubMed] [Google Scholar]
- Wilson R. H., Evans H. J., Becker R. R. The effect of univalent cation salts on the stability and on certain physical properties of pyruvate kinase. J Biol Chem. 1967 Sep 10;242(17):3825–3832. [PubMed] [Google Scholar]
- Wood T. The inhibition of pyruvate kinase by ATP. Biochem Biophys Res Commun. 1968 Jun 10;31(5):779–785. doi: 10.1016/0006-291x(68)90630-x. [DOI] [PubMed] [Google Scholar]


