Abstract
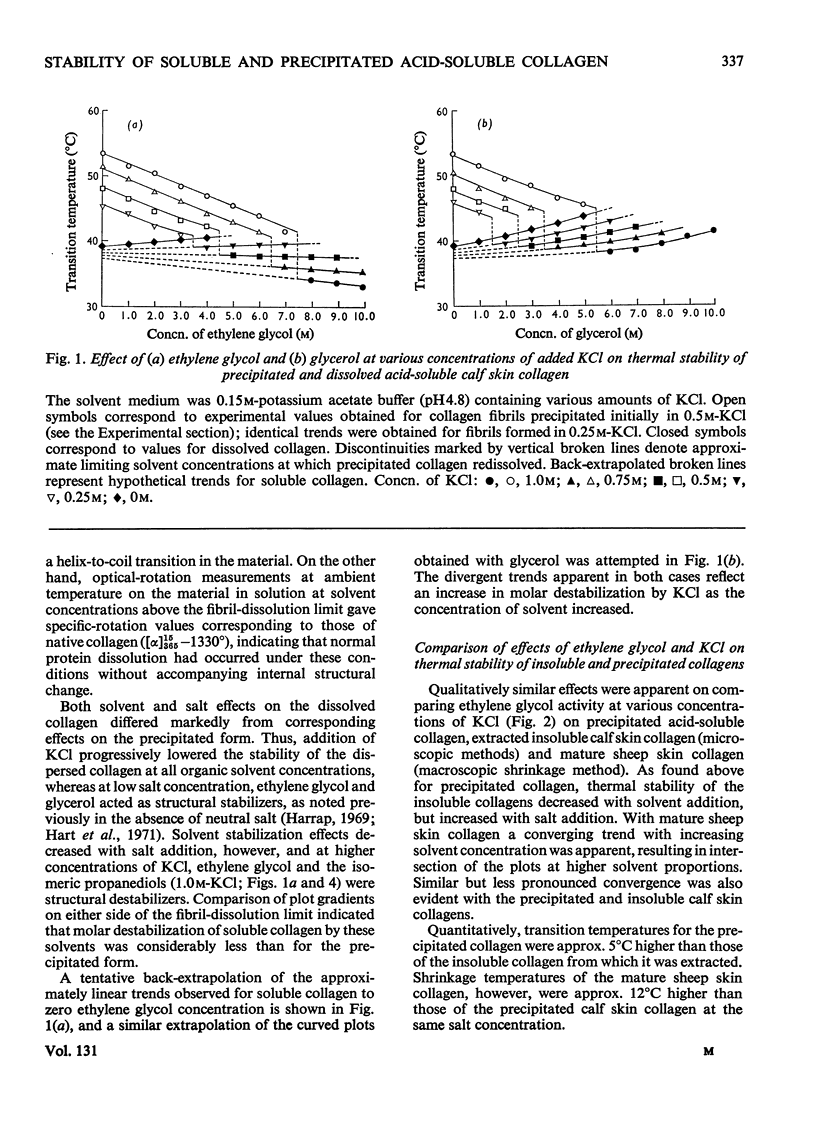
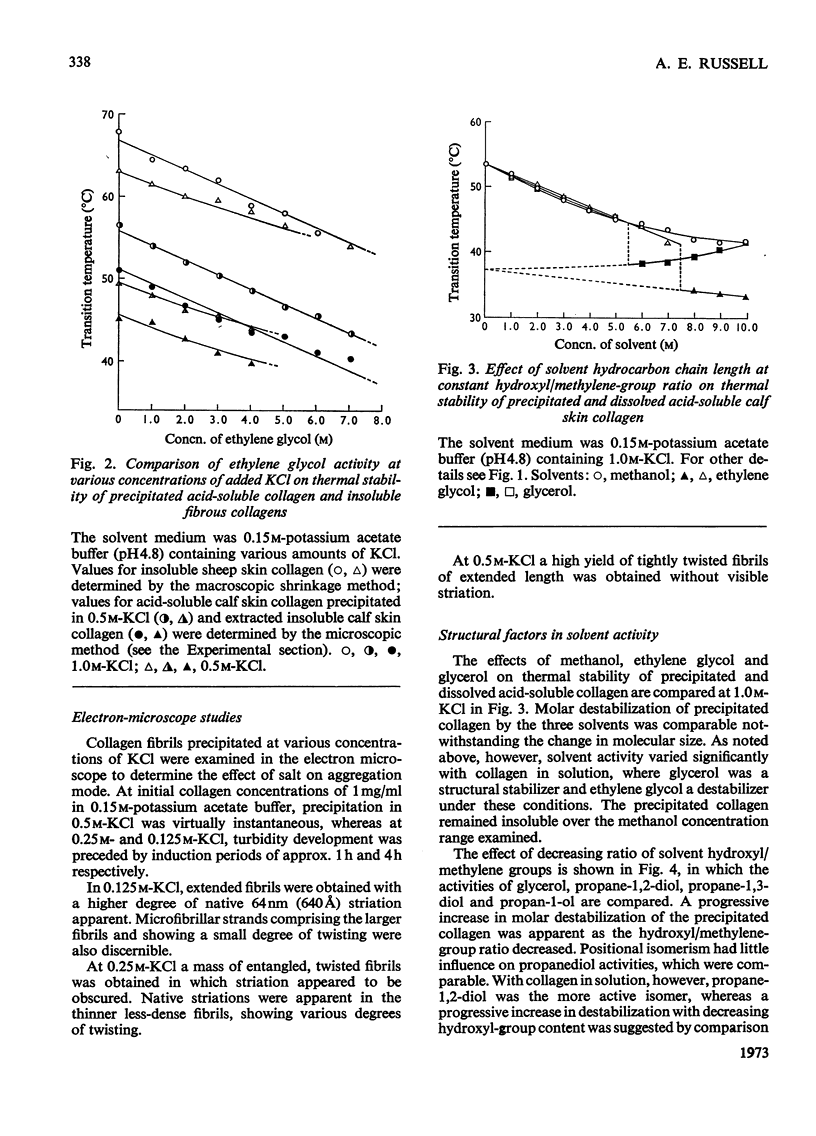
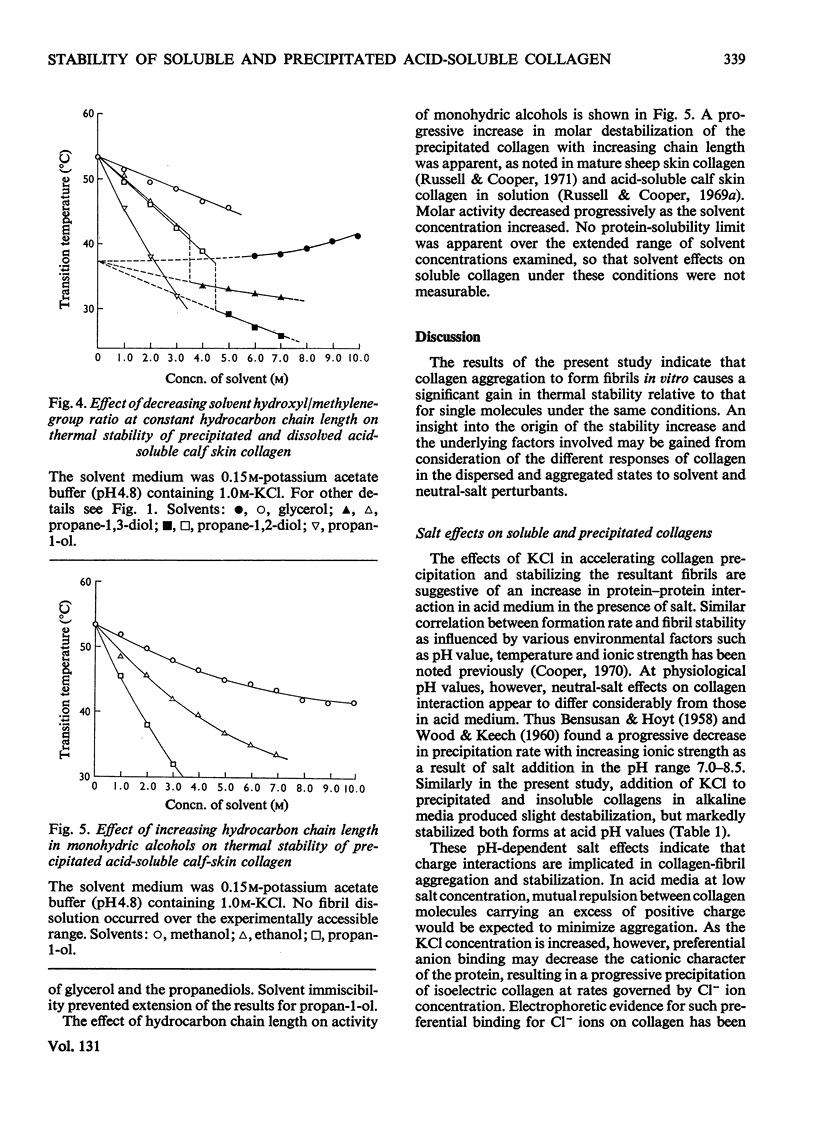
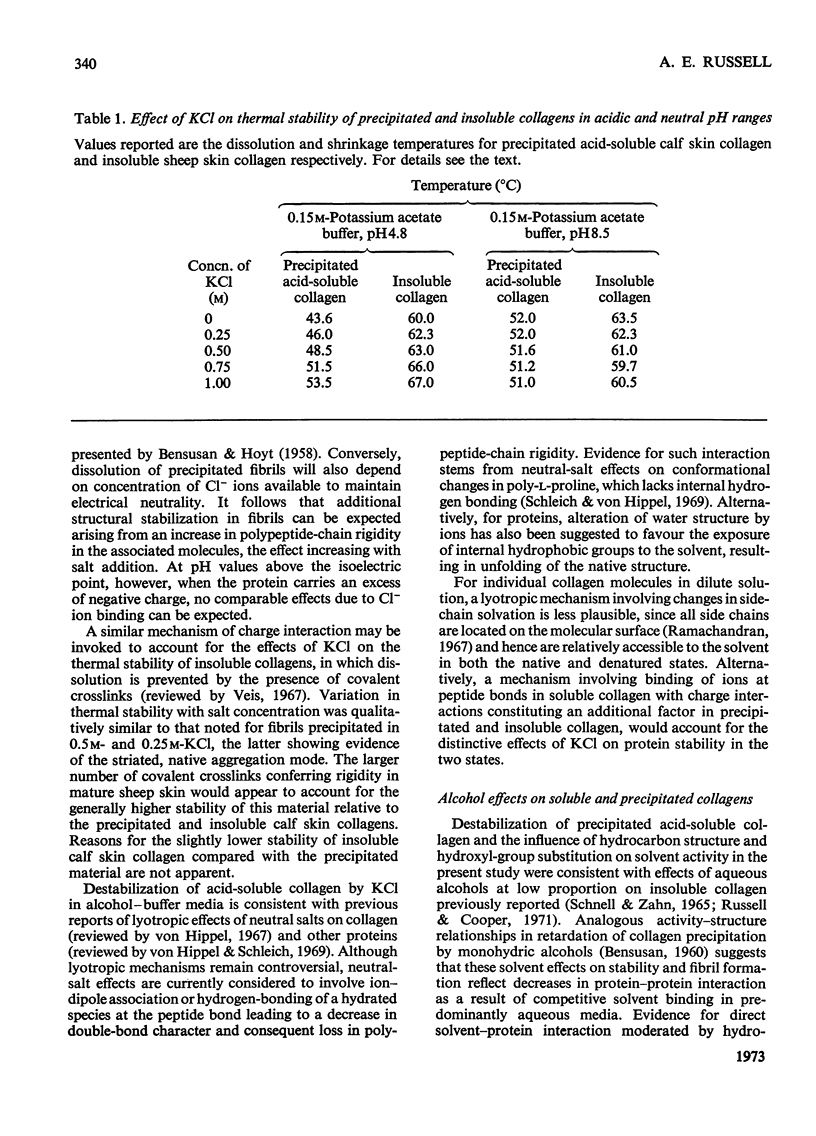
The effects of mono- and poly-hydric alcohols in the presence of KCl on the intrinsic stability of collagen molecules in dilute acid solution were compared with corresponding solvent and salt effects on the increased stability of the aggregated molecules in salt-precipitated fibrils. Salt addition decreased solubility and increased the thermal stability of fibrils, but progressively decreased the stability of collagen molecules in solution. In contrast, the alcohols enhanced solubility and decreased fibril stability, the effects increasing with solvent hydrocarbon chain length and with decreasing hydroxyl/methylene-group ratio. Molar destabilization of dissolved collagen by alcohols was lower than for fibrils, and at low salt concentration, both ethylene glycol and glycerol were structural stabilizers. Electron-micrograph studies indicated that salt-precipitated fibrils tended to adopt the native aggregation mode, and qualitatively similar solvent effects were observed in insoluble collagens. Implications of the experimental findings are discussed in terms of a model in which electrostatic and apolar interactions mainly govern the excess of stability in collagen fibrils whereas intrinsic stability of single molecules is a function of polar interactions and polypeptide-chain rigidity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchi E., Rampone R., Tealdi A., Ciferri A. The role of aliphatic alcohols on the stability of collagen and tropocollagen. J Biol Chem. 1970 Jul 10;245(13):3341–3345. [PubMed] [Google Scholar]
- Cooper A. Thermal stability of tropocollagens--are hydrogen bonds really important? J Mol Biol. 1971 Jan 14;55(1):123–127. doi: 10.1016/0022-2836(71)90287-7. [DOI] [PubMed] [Google Scholar]
- Cooper A. Thermodynamic studies of the assembly in vitro of native collagen fibrils. Biochem J. 1970 Jul;118(3):355–365. doi: 10.1042/bj1180355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. R., Davidson R. J. The effect of ultraviolet irradiation on soluble collagen. Biochem J. 1965 Oct;97(1):139–147. doi: 10.1042/bj0970139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. R., Russell A. E., Hart G. J. The effects of glycols on the renaturation of soluble collagen. Biochem J. 1971 Dec;125(4):1069–1074. doi: 10.1042/bj1251069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J. [Studies on the formation of collagen. III. Time-dependent solubility changes of collagen in vitro]. J Exp Med. 1958 Aug 1;108(2):215–226. doi: 10.1084/jem.108.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrap B. S. The effect of aliphatic alcohols on the thermal stability of tropocollagen under acidic conditions. Int J Protein Res. 1969;1(4):245–252. doi: 10.1111/j.1399-3011.1969.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Hart G. J., Russell A. E., Cooper D. R. The effects of certain glycols, substituted glycols and related organic solvents on the thermal stability of soluble collagen. Biochem J. 1971 Nov;125(2):599–604. doi: 10.1042/bj1250599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbage D., Hanus A., Vallet G. Stabilité thermique du collagène acido-soluble dans des mélanges eau-solvants organiques. Biochim Biophys Acta. 1968 Dec 3;168(3):544–554. [PubMed] [Google Scholar]
- Herskovits T. T., Gadegbeku B., Jaillet H. On the structural stability and solvent denaturation of proteins. I. Denaturation by the alcohols and glycols. J Biol Chem. 1970 May 25;245(10):2588–2598. [PubMed] [Google Scholar]
- Herskovits T. T., Jaillet H., DeSena T. On the structural stability and solvent denaturation of proteins. 3. Denaturation by the amides. J Biol Chem. 1970 Dec 25;245(24):6511–6517. [PubMed] [Google Scholar]
- Herskovits T. T., Jaillet H., Gadegbeku B. On the structural stability and solvent denaturation of proteins. II. Denaturation by the ureas. J Biol Chem. 1970 Sep 10;245(17):4544–4550. [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Kaminsky L. S., Yong F. C., King T. E. Circular dichroism studies of the perturbations of cytochrome c by alcohols. J Biol Chem. 1972 Mar 10;247(5):1354–1359. [PubMed] [Google Scholar]
- Privalov P. L., Tiktopulo E. I. Thermal conformational transformation of tropocollagen. I. Calorimetric study. Biopolymers. 1970 Feb;9(2):127–139. doi: 10.1002/bip.1970.360090202. [DOI] [PubMed] [Google Scholar]
- Russell A. E., Cooper D. R. Comparison of lyotropic and chromatographic effects of polar organic solvents on collagen and cellulose. Biochemistry. 1971 Oct 12;10(21):3890–3896. doi: 10.1021/bi00797a015. [DOI] [PubMed] [Google Scholar]
- Russell A. E., Cooper D. R. Effect of compounds of the urea-guanidinium class on renaturation and thermal stability of acid-soluble collagen. Biochem J. 1972 May;127(5):855–863. doi: 10.1042/bj1270855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. E., Cooper D. R. Enhanced collagen renaturation in the presence of a lyotropic agent. Biochem J. 1969 Jun;113(1):221–223. doi: 10.1042/bj1130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. E., Cooper D. R. Structural and functional factors in the hydrogen bonding of polar organic solvents to acid-soluble collagen. Effect on renaturation kinetics and thermal stability. Biochemistry. 1969 Oct;8(10):3980–3990. doi: 10.1021/bi00838a014. [DOI] [PubMed] [Google Scholar]
- Russell A. E., Cooper D. R. Structural and functional factors in the lyotropic activity of amides and alkyl-substituted amides on acid-soluble collagen. Biochemistry. 1970 Jul 7;9(14):2802–2806. doi: 10.1021/bi00816a008. [DOI] [PubMed] [Google Scholar]
- SCHRIER E. E., SCHERAGA H. A. The effect of aqueous alcohol solutions on the thermal transition of ribonuclease. Biochim Biophys Acta. 1962 Oct 22;64:406–408. doi: 10.1016/0006-3002(62)90752-7. [DOI] [PubMed] [Google Scholar]
- Schnell J. Evidence for the existence of hydrophobic interactions as a stabilizing factor in collagen structure. Arch Biochem Biophys. 1968 Sep 20;127(1):496–502. doi: 10.1016/0003-9861(68)90254-3. [DOI] [PubMed] [Google Scholar]
- Strassmair H., Knof S., Engel J. Different binding of solvent to the peptide carbonyl group in different conformational environments induces the helix I is formed from helix II transition of poly L-proline. Hoppe Seylers Z Physiol Chem. 1969 Sep;350(9):1153–1154. [PubMed] [Google Scholar]
- Von Hippel P. H., Wong K. Y. On the conformational stability of globular proteins. The effects of various electrolytes and nonelectrolytes on the thermal ribonuclease transition. J Biol Chem. 1965 Oct;240(10):3909–3923. [PubMed] [Google Scholar]
- WOOD G. C., KEECH M. K. The formation of fibrils from collagen solutions. 1. The effect of experimental conditions: kinetic and electron-microscope studies. Biochem J. 1960 Jun;75:588–598. doi: 10.1042/bj0750588. [DOI] [PMC free article] [PubMed] [Google Scholar]


