Abstract
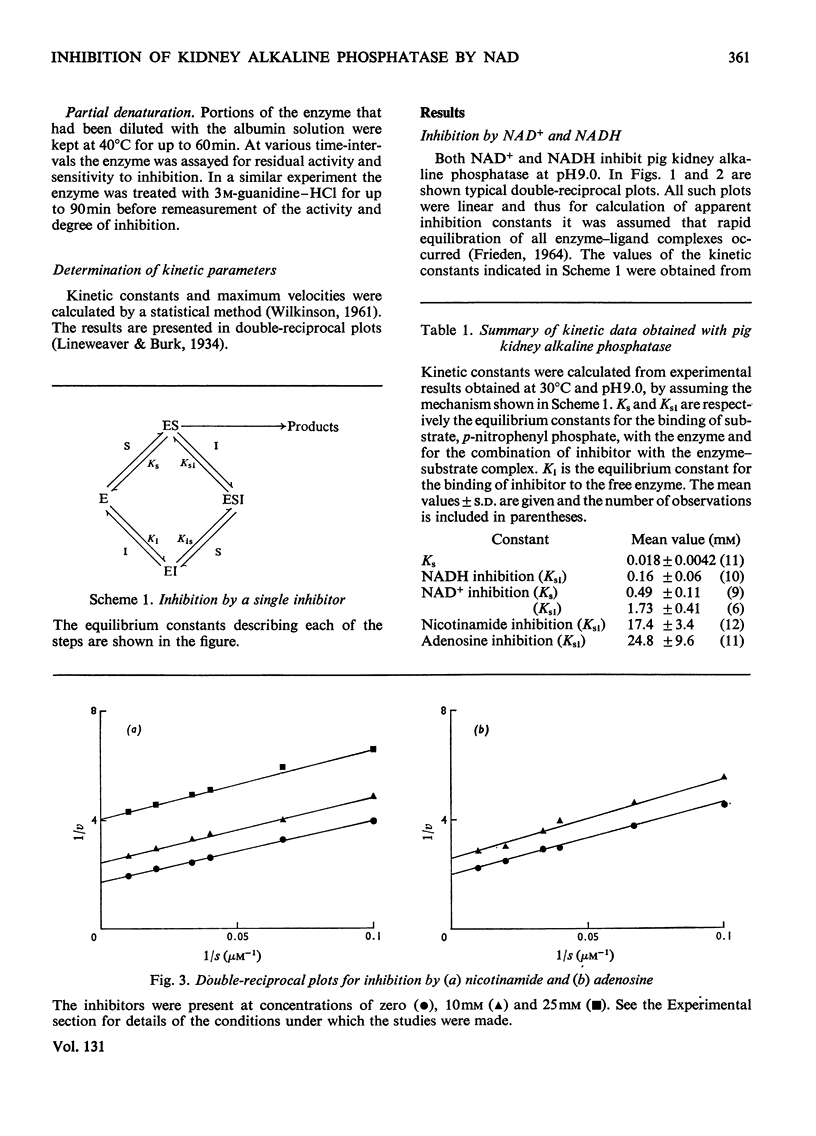
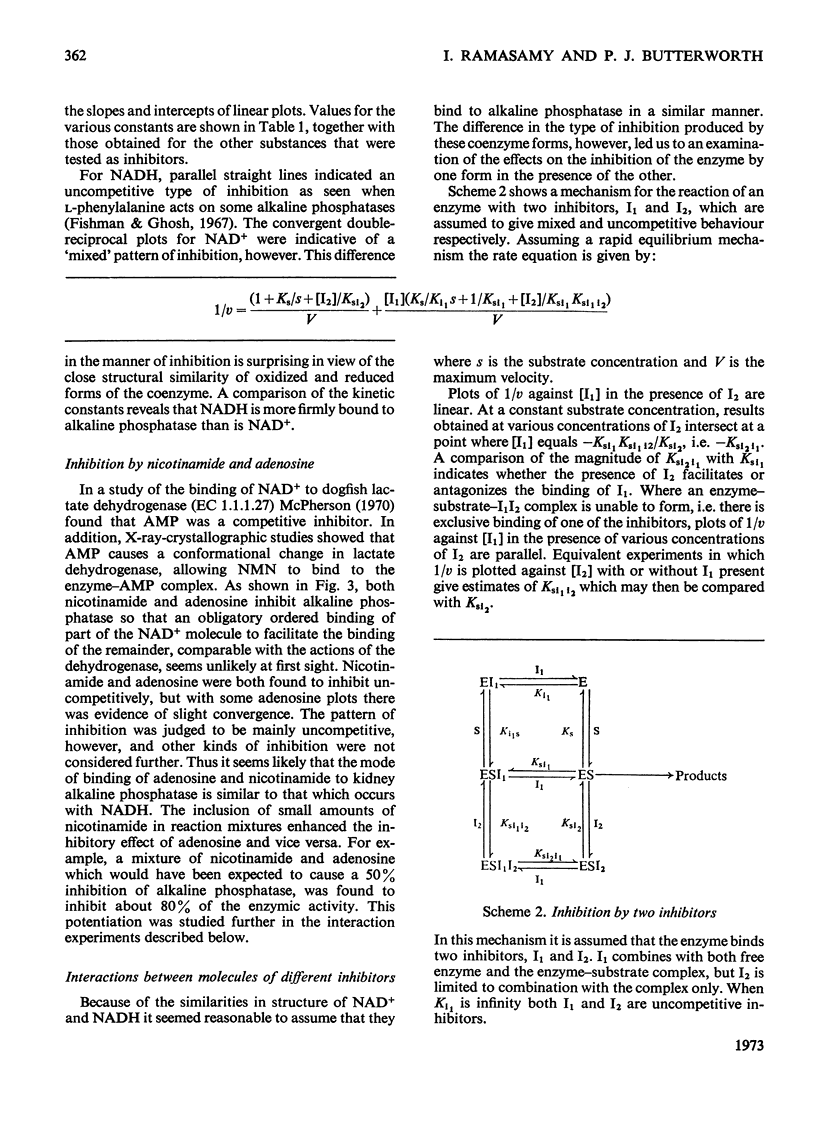
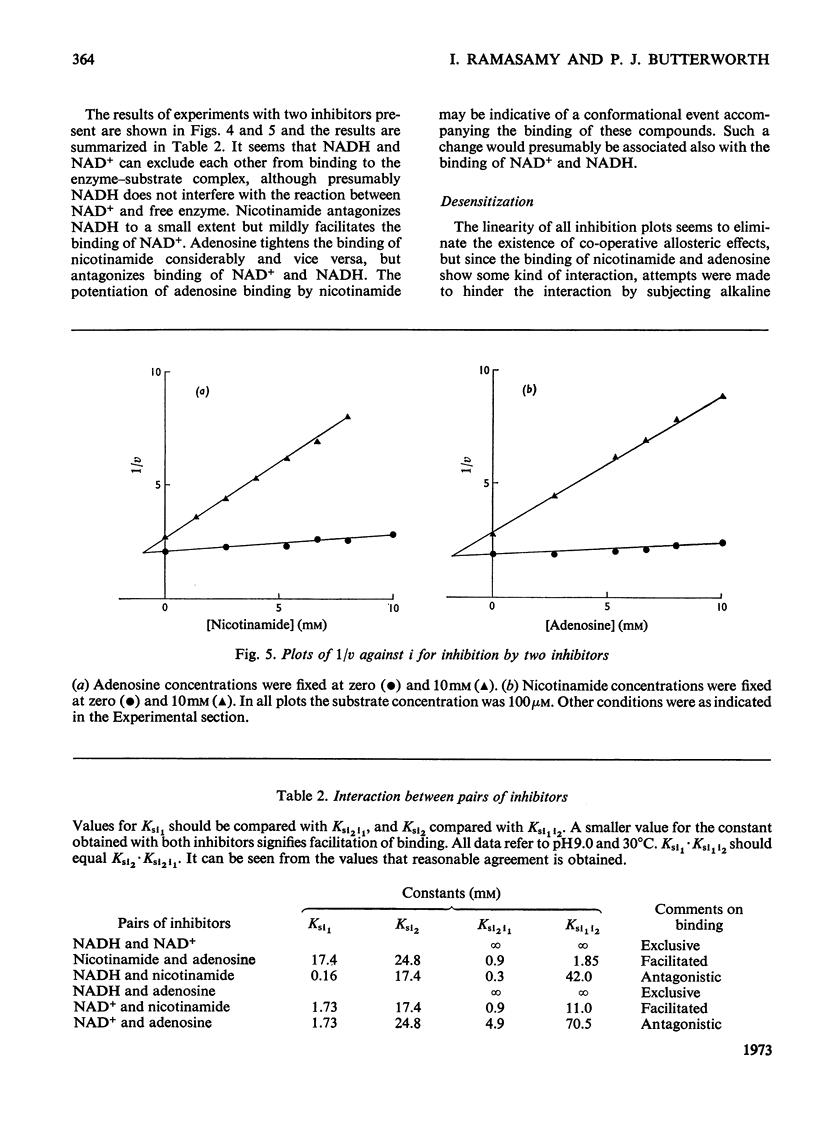
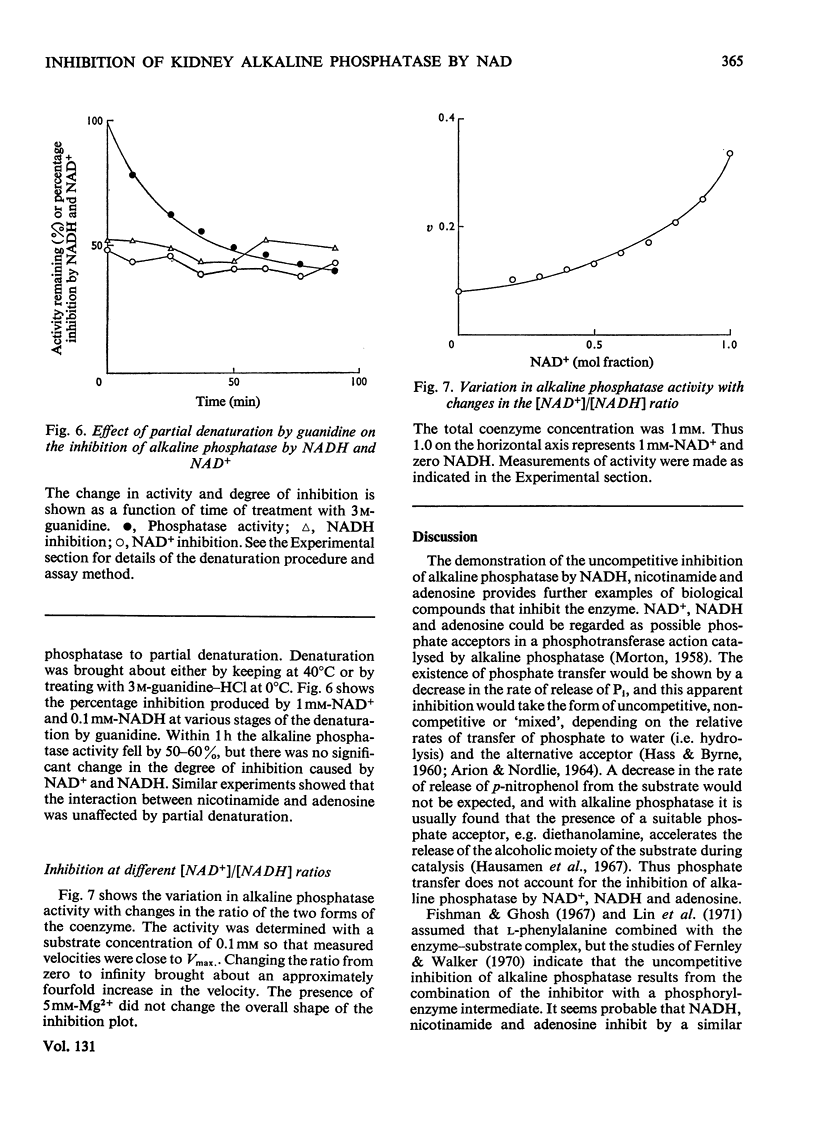
1. The inhibition of alkaline phosphatase by NAD+, NADH, adenosine and nicotinamide was studied. 2. All of these substances except NAD+ act as uncompetitive inhibitors, i.e. double-reciprocal plots are parallel. NAD+, however, is a `mixed' inhibitor of alkaline phosphatase and is less potent than NADH. 3. Inhibition studies with pairs of the inhibitors suggest that, in spite of the difference in type of inhibition, NAD+ and NADH bind to alkaline phosphatase at a common site. Adenosine and nicotinamide also seem to bind at the NAD site and the binding of adenosine is facilitated by nicotinamide, and vice versa. 4. The facilitation may indicate the occurrence of an induced fit for NAD+ and NADH. Attempts to desensitize alkaline phosphatase to NAD+ and NADH inhibition by partial denaturation were unsuccessful. 5. The results are discussed in terms of a two-site model in which separate, but interacting, regions exist on the enzyme to accommodate the adenosine and nicotinamide moieties of NAD, and a single-site model in which the adenosine part of the molecule is bound preferentially and this interacts with the nicotinamide fraction. 6. The activity of alkaline phosphatase can be changed fourfold by alteration of the NAD+/NADH ratio. This sensitivity to the redox state of the coenzyme could be a means of controlling phosphatase activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARION W. J., NORDLIE R. C. LIVER MICROSOMAL GLUCOSE 6-PHOSPHATASE, INORGANIC PYROPHOSPHATASE, AND PYROPHOSPHATE-GLUCOSE PHOSPHOTRANSFERASE. II. KINETIC STUDIES. J Biol Chem. 1964 Sep;239:2752–2757. [PubMed] [Google Scholar]
- Blumenstein M., Raftery M. A. 31 P and 13 C nuclear magnetic resonance studies of nicotinamide-adenine dinucleotide and related compounds. Biochemistry. 1972 Apr 25;11(9):1643–1648. doi: 10.1021/bi00759a017. [DOI] [PubMed] [Google Scholar]
- Butterworth P. J. The reversible inactivation of pig kidney alkaline phosphatase at low pH. Biochem J. 1968 Jun;108(2):243–246. doi: 10.1042/bj1080243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. H., Moss D. W. Organic pyrophosphates as substrates for human alkaline phosphatases. Biochem J. 1967 Dec;105(3):1307–1312. doi: 10.1042/bj1051307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHMAN W. H., GREEN S., INGLIS N. I. L-phenylalanine: an organ specific, stereospecific inhibitor of human intestinal alkaline phosphatase. Nature. 1963 May 18;198:685–686. doi: 10.1038/198685b0. [DOI] [PubMed] [Google Scholar]
- FISHMAN W. H., GREEN S., INGLIS N. I. Organ-specific behavior exhibited by rat intestine and liver alkaline phosphatase. Biochim Biophys Acta. 1962 Aug 13;62:363–375. doi: 10.1016/0006-3002(62)90266-4. [DOI] [PubMed] [Google Scholar]
- FRIEDEN C. TREATMENT OF ENZYME KINETIC DATA. I. THE EFFECT OF MODIFIERS ON THE KINETIC PARAMETERS OF SINGLE SUBSTRATE ENZYMERS. J Biol Chem. 1964 Oct;239:3522–3531. [PubMed] [Google Scholar]
- Fernley H. N., Walker P. G. Inhibition of alkaline phosphatase by L-phenylalanine. Biochem J. 1970 Feb;116(3):543–544. doi: 10.1042/bj1160543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher H. F., Gates R. E., Cross D. G. A ligand exclusion theory of allosteric effects. Nature. 1970 Oct 17;228(5268):247–249. doi: 10.1038/228247a0. [DOI] [PubMed] [Google Scholar]
- Fishman W. H., Sie H. G. Organ-specific inhibition of human alkaline phosphatase isoenzymes of liver, bone, intestine and placenta; L-phenylalanine, L-tryptophan and L homoarginine. Enzymologia. 1971 Sep 30;41(3):141–167. [PubMed] [Google Scholar]
- Ghosh N. K., Fishman W. H. L-phenylalanine inhibiton of rat intestinal alkaline phosphatase: a homosteric phenomenon. Arch Biochem Biophys. 1968 Aug;126(2):700–706. doi: 10.1016/0003-9861(68)90457-8. [DOI] [PubMed] [Google Scholar]
- Ghosh N. K., Fishman W. H. On the mechanism of inhibition of intestinal alkaline phosphatase by L-phenylalanine. I. Kinetic studies. J Biol Chem. 1966 Jun 10;241(11):2516–2522. [PubMed] [Google Scholar]
- Gumaa K. A., McLean P., Greenbaum A. L. Compartmentation in relation to metabolic control in liver. Essays Biochem. 1971;7:39–86. [PubMed] [Google Scholar]
- Haussler M., Nagode L. A., Rasmussen H. Induction of intestinal brush border alkaline phosphatase by vitamin D and identity with ca-ATPase. Nature. 1970 Dec 19;228(5277):1199–1201. doi: 10.1038/2281199a0. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A. Active sugar accumulation by isolated intestinal epithelial cells. A new model for sodium-dependent metabolite transport. Biochemistry. 1970 Sep 15;9(19):3669–3677. doi: 10.1021/bi00821a004. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., ROCK M. K. The stability of pyridine nucleotides. J Biol Chem. 1961 Oct;236:2756–2759. [PubMed] [Google Scholar]
- Lin C. W., Sie H. G., Fishman W. H. L-tryptophan. A non-allosteric organ-specific uncompetitive inhibitor of human placental alkaline phosphatase. Biochem J. 1971 Sep;124(3):509–516. doi: 10.1042/bj1240509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. The phosphotransferase activity of phosphatases. 2. Studies with purified alkaline phosphomonoesterases and some substrate-specific phosphatases. Biochem J. 1958 Sep;70(1):139–150. doi: 10.1042/bj0700139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson A., Jr Interaction of lactate dehydrogenase with its coenzyme, nicotinamide-adenine dinucleotide. J Mol Biol. 1970 Jul 14;51(1):39–46. doi: 10.1016/0022-2836(70)90268-8. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Mircheff A. K., Adams T. H., Spielvogel A. Studies on the mechanism of action of calciferol. 3. Vitamin D-mediated increase of intestinal brush order alkaline phosphatase activity. Biochim Biophys Acta. 1970 Aug 14;215(2):348–359. doi: 10.1016/0304-4165(70)90034-6. [DOI] [PubMed] [Google Scholar]
- Sarma R. H., Kaplan N. O. High frequency nuclear magnetic resonance investigation of the backbone of oxidized and reduced pyridine nucleotides. Biochemistry. 1970 Feb 3;9(3):557–564. doi: 10.1021/bi00805a015. [DOI] [PubMed] [Google Scholar]
- Sarma R. H., Kaplan N. O. High frequency nuclear magnetic resonance study of the M and P helices of reduced pyridine dinucleotides. Biochemistry. 1970 Feb 3;9(3):539–548. doi: 10.1021/bi00805a013. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Raijman L., Krebs H. A. Equilibrium relations between the cytoplasmic adenine nucleotide system and nicotinamide-adenine nucleotide system in rat liver. Biochem J. 1970 Apr;117(3):499–503. doi: 10.1042/bj1170499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass M., Butterworth P. J. Nucleoside pyrophosphatase activity associated with pig kidney alkaline phosphatase. Biochem J. 1971 Oct;124(5):891–896. doi: 10.1042/bj1240891. [DOI] [PMC free article] [PubMed] [Google Scholar]


