Summary
Background
mRNA-based cancer vaccines show promise in triggering antitumour immune responses. To combine them with existing immunotherapies, the intratumoral immune microenvironment needs to be deeply characterised. Here, we test nanostructured lipid carriers (NLCs), the so-called Lipidots®, for delivering unmodified mRNA encoding Ovalbumin (OVA) antigen to elicit specific antitumour responses.
Methods
We evaluated whether NLC OVA mRNA complexes activate dendritic cells (DCs) in vitro and identified the involved signalling pathways using specific inhibitors. We tested the anti-tumoral impact of Ova mRNA vaccine in B16-OVA and E.G7-OVA cold tumour-bearing C57Bl6 female mice as well as its synergy effect with an anti-PD-1 therapy by following the tumour growth and performing immunophenotyping of innate and adaptive immune cells. The intratumoral vaccine-related gene signature was assessed by RNA-sequencing. The immune memory response was assessed by re-challenging surviving treated mice with tumour cells.
Findings
Our vaccine activates DCs in vitro through the TLR4/8 and ROS signalling pathways and induces specific T cell activation while exhibits significant preventive and therapeutic antitumour efficacy in vivo. A profound intratumoral remodelling of the innate and adaptive immunity in association with an increase in the gene expression of chemokines (Cxcl10, Cxcl11, Cxcl9) involved in CD8+ T cell attraction were observed in immunised mice. The combination of vaccine and anti-PD-1 therapy improves the rates of complete responses and memory immune responses compared to monotherapies.
Interpretation
Lipidots® are effective platform for the development of vaccines against cancer based on mRNA delivery. Their combination with immune checkpoint blockers could counter tumour resistance and promote long-term antitumour immunity.
Funding
This work was supported by Inserm Transfert, la Région Auvergne Rhône Alpes, FINOVI, and the French Ministry of Higher Education, research and innovation (LipiVAC, COROL project, funding reference N° 2102992411).
Keywords: mRNA vaccine, Immune checkpoint inhibitor, Cancer, T cells, Innate immunity, Memory immunity
Research in context.
Evidence before this study
Impact of anticancer mRNA vaccines has been mostly assessed peripherally, with limited intratumoral analysis despite expected use with immune checkpoint inhibitors. Tumour escape mechanisms and long-term memory response to mRNA vaccines are not well understood. We tested Lipidots® lipid nanoparticles for mRNA vaccines in tumour-bearing mice, focussing on intratumoral immune investigations and re-challenge experiments.
Added value of this study
We found that vaccination alone reduces tumour growth by promoting a T cell–inflamed tumour microenvironment but also drives PD-1/PDL-1 escape mechanism. Combining mRNA-based vaccine with anti-PD-1 therapy increases the rate of total tumour regression and resistance to re-challenge, while enhancing early granzyme B production by intratumoral CD4+ T cells when compared to the control group.
Implications of all the available evidence
Results from this study suggest that mRNA vaccines can be an adjuvant to immune checkpoint inhibitors (ICI) in cold tumours, while ICI enhances the long-term efficacy of antitumour mRNA vaccines. This underscores the need to prioritise combination therapy over monotherapy in clinical testing for patients with cancer.
Introduction
The combination of immunosuppression in the tumour microenvironment (TME) and low immunogenicity of cancer cells are important features that hinders effective immune-mediated tumour regression. Tumours explore a variety of mechanisms to evade immune detection including expression of immune inhibitory ligands such as programmed death ligand 1 (PD-L1), repression of tumour antigen presentation and release of immunosuppressive molecules such as TGF-β [1]. To rearm an effective antitumoral immune response, immune checkpoint blockade (ICB), which notably targets the key signalling axis involving programmed death-1 (PD-1) and its ligands PD-L1 and PD-L2, is the most commonly used immunotherapy in patients with cancer and demonstrates remarkable beneficial effects in various solid malignancies [2, 3, 4, 5]. Despite its clinical success, many patients do not respond optimally to ICB due to primary or acquired resistance [6]. Complementary therapeutic strategies that aim to make the tumour hotter by stimulating T-effector-cell infiltration are needed to maximise the clinical effectiveness of ICB [7, 8, 9].
The impairment of naive T cell priming caused by the activation of cancer cell-specific oncogenic pathways, which leads to ineffective intratumoral recruitment of dendritic cells (DCs), is associated with ICB resistance in melanoma and hepatocellular cancer models [10,11]. In weakly immunogenic and/or immune desert tumours, therapeutic cancer vaccine by eliciting a specific T cell response that promotes intratumoral T-cell trafficking and because of its low toxicity would be a leading treatment to be combined with ICB [12, 13, 14]. With the approval of COVID-19 mRNA vaccines using first generation of lipid nanoparticle (LNP) delivery platforms by US FDA, the safety and efficacy of this therapeutic technology were broadly demonstrated [15,16]. Importantly, mRNA-based cancer vaccines have several advantages including their good tolerability, non-infectious composition, in addition to their fast and low-cost production [17]. Among the diverse nanocarrier pharmaceutical systems, lipid-based nanodelivery systems have become the most common used vectors for mRNA cancer vaccine because of high mRNA preservation and cellular uptake [18]. Following administration, LNP-mRNA based vaccine is mainly internalised by antigen presenting cells (APCs), including DCs, which translate the mRNA-encoding tumour-associated antigen while undergoing maturation and activation that benefits the subsequent generation of the adaptive immunity [19,20]. Although this therapy has not yet been approved for standard treatment in oncology, multiple clinical trials of mRNA vaccines are currently on going [13] and encouraging results have already been obtained for melanoma and pancreatic cancer [21,22]. Several preclinical studies have demonstrated the antitumour efficacy of vaccines delivering mRNA coding for OVA or tumour antigens [23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33], however, they have rarely explored the reshaping of the tumour immune microenvironment, focussing more on evaluating the peripheral response, or the anticancer memory immunity. Since these two aspects are essential for understanding the mechanisms of sensitivity and resistance to immunotherapies, we particularly addressed them.
We have previously developed nanostructured lipid carriers (NLCs), so called Lipidots®, formulated with biocompatible and FDA-approved ingredients (wax, oil, lecithin, PEG-stearate) that have been extensively characterised for physicochemical aspects [34, 35, 36, 37]. This NLCs, has a natural tropism toward lymph nodes [38] and are captured by DCs from mesenteric lymph nodes following an intraperitoneal injection [36]. This NLC formulation has been adapted through a design of experiments approach by incorporating cationic and fusogenic lipids while preserving their core/shell structure and colloidal stability, thus enabling the loading of negatively charged nucleic acids through electrostatic interactions. We have therefore demonstrated in vitro the intracellular delivery of siRNA or oligonucleotides into bacteria, fibroblasts, DCs, and macrophages in prior studies [39, 40, 41]. Based on that, we investigated whether NLC- unmodified mRNA coding ovalbumin (OVA) complex can be transfected in primary murine DCs and trigger an OVA specific T cell response. We also evaluated its capacity to induce a specific antitumoral immune response in murine melanoma and lymphoma models expressing OVA (B16-OVA and E.G7-OVA) and to improve the effectiveness of anti-PD-1 blocking antibody in B16-OVA tumours which are poorly infiltrated with immune cells and resistant to anti-PD-1 therapy. First, we show that our mRNA-based vaccine strategy activates DCs through Toll-Like Receptor (TLR) and reactive oxygen species (ROS) signalling in vitro and is efficiently translated and processed to induce OVA-specific CD8+ and CD4+ T cell activations. Subsequently, we demonstrate that NLC-Ova mRNA-based vaccine decreases tumour growth in association with the promotion of a T cell–inflamed tumour microenvironment, characterised by active IFN-γ signalling, cytotoxic effector molecules and antigen presentation that are all essential features for anti-PD-1 responsiveness [8,42]. Combining mRNA-based vaccine with anti-PD-1 increases the early production of granzyme B by intratumoral CD4+ T cells, improves survival and leads to a higher rate of total tumour regression. Finally, mRNA-based vaccine ± anti-PD-1- treated mice surviving the first B16-OVA tumour engraftment are totally resistant to tumour re-challenge expressing OVA (B16-OVA) while partially resistant when tumour cells do not express OVA (B16) implying the development of immunologic memory and suggesting antigen spreading.
Methods
Mice and ethical statement
Wild type C57BL/6 mice were purchased from Charles River laboratories (Saint-Germain sur l'Arbresle, France). OT-I and OT-II mice were bred at «Plateforme de Haute Technologie Animale (PHTA)» University of Grenoble-Alpes core facility (Grenoble, France). Female mice greater than 9 weeks of age were matched by age and randomly attributed to the different treatment groups. The number of mice in each treated group for each experiment is indicated in the legend of the figures. No mice were excluded based on preestablished criteria, and randomisation was applied only immediately pretreatment to ensure similar mean tumour size at the start of therapy experiments. All animals were maintained in specific pathogen free facility at the animal facility of the Institute for Advanced Biosciences. Animal use and care were approved by the Animal Experiments Ethics Committee “Comité d'Ethique pour l'Expérimentation Animale no. #12, Cometh-Grenoble” and approved by the French Ministry of Research (#32695-2021081311583272 v3). All experiments were performed in accordance with ARRIVE guidelines.
C.F. was responsible for the experimental operations, A.A., C.C., A.L., V.D., D.R., J.V., C.F., and BGI genomics performed the measurements, C.F., S.E., and J.V. made the data analysis. The primary outcome measure was the tumour size and this parameter was used for the sample size calculation.
Cell culture
B16 and B16-OVA (RRID: CVCL_F936) mouse melanoma cell lines were kindly provided by Sandrine Henri (Centre d'Immunologie Marseille-Luminy (CIML), Marseille, France) and Alvaro Baeza Garcia (Centre de recherche Translationnelle en Médecine moléculaire, UMR1231, Dijon, France), respectively. E.G7-OVA cell line (lymphoma, RRID: CVCL_3505) was purchased from ATCC (#CRL-2113). All cell lines were maintained in humidified air with 5% CO2 at 37 °C in the appropriate culture media. Cell lines were grown in Roswell Park Memorial Institute (RPMI) 1640 w/l-glutamine supplemented with 10% (vol/vol) heat-inactivated foetal bovine serum (FBS), 1 mM Sodium Pyruvate (Gibco) and 1% Penicillin Streptomycin (P/S). B16-OVA and B16 media were completed with 1% Non-Essential Amino Acids (NEAA) and E.G7-OVA media with 10 mM HEPES, 0.05 mM 2-mercaptoethanol, and 0.4 mg/ml G418 (Gibco). None of the cell lines used in this study are listed by the International Cell Line Authentication Committee as cross-contaminated or misidentified cell lines (v13, 2024). The cell lines were tested to be mycoplasma negative (Lonza, MycoAlert® PLUS Mycoplasma Detection Kit) and the number of passages did not exceed 11.
For bone marrow derived DCs (BMDCs), bone marrow was isolated from the tibias and femurs of female C57BL/6 mice (6 weeks old) and processed into a single cell suspension as described in a previous study [43]. GR1 positive cells and erythrocytes were removed following incubation with Ly-6G/Ly-6C (BD Pharmingen, #553125, RRID: AB_394641) and TER-119 (BD Pharmingen, #553672, RRID: AB_394985) antibodies, and the remaining negatively sorted cells were isolated using Dynabeads isolation kit (ThermoFisher Scientific, #11047) by magnetic cell sorting. Then, the sorted cells were cultured at 5 × 105 cells/ml into a 100-mm standard tissue culture dish (Falcon™ #353003) with 15 ml of complete Iscove's modified Dulbecco's medium (IMDM containing 10% heat-inactivated FBS, 1% NEAA, 1% sodium pyruvate, 0.1% 2-mercaptoethanol, and 1% P/S) supplemented with GM-CSF (PeproTech, #315-03), FLT-3L (PeproTech, #250-31L), and IL-6 (PeproTech, #216-16) according to the Supplemental Table S1. After 10 days of expansion, BMDCs were collected by centrifugation, resuspended in the complete IMDM media and used for experiments.
For coculture experiments, CD4 or CD8 T cells were purified from the spleen of OT-II or OT-I mice respectively. Briefly, spleen was mechanically dissociated through a 100 μM cell strainer (Falcon®), cells were centrifuged and red blood cells were lysed. After washing, cells were isolated by negative magnetic sorting using a Dynabeads™ Untouched™ Mouse CD4 or CD8 Cells kit (Invitrogen #11415D #11417D) according to the manufacturer's instructions. Cells were resuspended in complete RPMI media (10% FBS, 1% NEAA, 1% sodium pyruvate, 1% P/S) and used immediately.
Nanostructured lipid carrier preparation
NLCs were prepared as described previously [36]. Briefly, a lipid phase was prepared containing triglycerides (Suppocire NB, Gattefossé and super-refined soybean oil, Croda Uniqema) and phospholipids (Lipoid SPC3, Lipoid) supplemented with the cationic lipid DOTAP (1,2-dioleoyl-3-trimethylammonium-propane chloride, Avanti Polar Lipids) and fusogenic lipid DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, Avanti Polar Lipids). A second aqueous phase containing the PEGylated surfactant PEG-40 Stearate (Myrj S40, Croda Uniqema) was prepared in phosphate buffered saline (PBS) (#806552, Sigma). Both aqueous and lipid phases were mixed together through high-frequency sonication. Purification of NLC was performed by extensive dialysis in 100 volumes of NLC buffer: 154 mM NaCl, 10 mM HEPES, and pH 7.4 using endotoxin-free ultra-pure water (TMS-011-A, Sigma) and 12–14 kDa MW cut-off membranes (ZelluTrans/Roth T3). Lastly, the NLC solution was sterilised by filtrating through a 0.22-μm Millipore membrane under aseptic conditions and stored at 4 °C until required.
Physical characterisation of NLCs
The hydrodynamic diameter and polydispersity index (PDI) of the NLCs were determined by dynamic light scattering, and the zeta potential was evaluated by electrophoretic light scattering using a NanoSizer equipment (Malvern). For zeta potential measurement, a solution of 1 mg/ml of NLCs prepared in water was complexed or not with Ova mRNA (N/P 6) while for hydrodynamic diameter and PDI assessments, the same 1 mg/ml NLC solution was adjusted to PBS 1× by adding 10% volume of 0.22 μm filtered PBS 10× (#AM9625, Invitrogen). Each sample was measured in triplicate and experiment was reproduce two times. The size, PDI and zeta potential of NLC and NLC-Ova mRNA (N/P6) are documented in Supplemental Table S2 (data are shown as mean ± SEM).
Complexation of NLCs with mRNA
NLCs were complexed with Cherry, Egfp or Ova mRNA (Tebu bio #040L-7203-100; #040L-7601-100; #040L-7610-100) in IMDM media. The required volumes of mRNA and NLCs were calculated for obtaining a N/P ratio of 6 (ratio of positively-chargeable polymer amine (N = nitrogen) groups to negatively-charged nucleic acid phosphate (P) groups). The NLCs and mRNA were gently homogenised by pipetting and kept for 10 min at room temperature before immediate use for downstream experiments. mRNA is capped using the CleanCap technology from TriLink and mimic a fully processed mature mRNA.
Complexation efficiency was quantified with Quant-it™ RiboGreen Assay (ThermoFisher Scientific, #R11490). RiboGreen dye binds to RNA and produce a fluorescent signal proportional to the RNA concentration and was measured using a standard curve of the selected Ova mRNA. Samples were loaded into the reading plate v/v with 1× TE buffer (10 mM Tris–HCl, 1 mM EDTA) ± triton 0.5% to get free mRNA or total mRNA measurement. Fluorescence measurements were collected using Tecan Sparkcyto Reader (ʎex: 480 nm; ʎem: 530 nm). Complexation efficiency was calculated by taking the ratio of complexed mRNA (total mRNA—free mRNA) to total mRNA. Each sample was performed in triplicate, and experiment was reproduced twice. Data (mean ± SEM) are presented in the Supplemental Table S3 and shown that 97% of Ova mRNA are complexed with NLCs in our conditions.
Cell incubation with NLC-mRNA complexations and inhibitors
For BMDC treatment, 0.3 M cells were seeded in 96 well plates in 200 μL of IMDM media deprived in FBS and P/S and containing GM-CSF (5 ng/ml) and FLT-3L (25 ng/ml). After an overnight incubation at 37 °C under 5% CO2, 20 μL of NLC, Ova mRNA or NLC-Ova mRNA complexation in IMDM was added in each corresponding well. 20 μL of IMDM media alone was used for the no treatment condition (vehicle). For some wells, after 6 h of transfection the media was changed and the assessment of cytokines in the cell supernatant was performed at day 1.
For the experiments with TLR inhibitors, BMDCs were pretreated for 3 h with either TLR4 inhibitor TAK-242 (InvivoGen, #tlr-cli95) at three different concentrations (1.25 μM, 2.5 μM, and 5 μM) or with the TLR8 inhibitor CU-CPT9a (2.5 μM, 5 μM, and 10 μM) (InvivoGen, #inh-cc9a) and incubated at 37 °C. For the experiments with the antioxidant drugs, the pretreatment with diphenyleneiodonium (DPI) (0.1 μM, 1 μM, and 10 μM), a ROS-dependent NOX inhibitor (Sigma–Aldrich, D2926) or with S3QEL2 (1 μM, 10 μM, and 30 μM), a mitochondrial ROS (mROS) inhibitor (Sigma–Aldrich, SML1554) lasted 1 h. The cell supernatants were collected after 24 h of NLC-Ova mRNA complex incubation.
For coculture experiments, 40,000 BMDCs treated with 125 ng of vectorised mRNA for 6 h were washed with IMDM media and 200 μL of CD4 or CD8 T cells at 550,000 cells/mL were added. Cocultures were incubated for 48 h at 37 °C under 5% CO2 before supernatant collection and cell analysis. Protein Transport Inhibitor cocktail (PTI, eBioscience) was added for the last 6 h of culture.
Murine tumour models and in vivo treatments
B16-OVA (2 × 105) or E.G7-OVA (4 × 105) cells were intradermally (i.d.) implanted into the flank of female C57BL/6 mice in a final volume of 100 μL of sterile PBS. Digital callipers were used to measure the perpendicular diameters of the tumours three times a week. In agreement with ethical guidelines, mice were euthanised for a maximum tumour size of 1900 mm3 or when tumour ulcerations were visible. Any unexpected adverse events were observed.
Preventive therapy
3 μg of vectorised Cherry or Ova mRNA prepared in IMDM were injected intraperitoneally (i.p.) (100 μL per mouse) at day 0, 3, and 8. At day 14, B16-OVA cells were inoculated and mice were sacrificed at day 30 for subsequent analysis. For long term experiment, B16-OVA were grafted at day 49.
Curative therapy
After B16-OVA or E.G7-OVA tumour engraftment (day 0), mice were injected i.p. with 3 μg of vectorised Cherry, Egfp or Ova mRNA prepared in IMDM at the intervals indicated in the figure legends and sacrificed at day 17.
For CD8 or NK cell depletions, anti-CD8β (clone 53-5.8, BioXCell) or anti-NK1.1 (clone PK136, BioXCell) or their respective isotype control antibody (clone HRPN or C1.18.4, BioXCell) was injected i.p. at 150 μg per mouse at day 2, 7, and 13 after B16-OVA cells engraftment. For PD-1 blockade, anti-PD-1 (clone RMP1-14, BioXCell) or its isotype control antibody (clone 2A3, BioXCell) was injected i.p. at 100 μg per mouse at day 7, 12, and 17 relative to B16-OVA cell inoculation. Immunisations with 3 μg of vectorised mRNA were performed at day 3, 6, and 9.
Cancer challenge
To challenge survival mice after tumour regression, a new B16-OVA or B16 tumour was engrafted in the contralateral site (2 × 105 cells in a final volume of 100 μL of sterile PBS).
In vivo biodistribution of NLC-Ova mRNA in healthy mice
The in vivo experiments were carried out in accordance with the animal experimentation project authorisation notified by the French Ministry of Education and Research under APAFiS number APAFIS# 33137.
Six healthy female C57BL/6 mice (8-week-old, Janvier Labs, Le Genest-Saint Isle, France) were injected i.p. with 100 μL of NLC-IR780 complexed with 3 μg of Ova mRNA (N/P 6). At 5 h and 24 h post injection, mice were sacrificed and their organs were isolated for direct ex vivo fluorescence measurement on the Pearl imaging system (LI-COR, ex: 785 nm, em: BP 800–820 nm). Fluorescence signals were measured by setting regions of interest on the organs and expressed in Relative Light Unit (RLU) per pixel. Ex vivo autofluorescence background was measured on images of tissue samples from non-injected mice. For the production of the fluorescent NLCs, IR 780-Oleyl dye diluted in ethanol was added to the lipid phase in order to get a concentration of 550 μM in the final NLC product.
Cytokine measurement
Cell culture supernatant or serum were analysed by ELISA for IL-6 (#88-7064, Invitrogen), TNFα (#88-7324, Invitrogen) or IFNγ (#88-8314, Invitrogen) according to the manufacturer's instructions.
Toxicity assessment
Toxicity was evaluated by measuring the lactate dehydrogenase (LDH) level in cell supernatants using the CytoTox-ONE™ Homogeneous Membrane Integrity Assay kit (Promega, G7892) according to the manufacturer's instructions. Briefly, cell lysis was induced with a lysis buffer and was used as positive control for LDH release. 50 μl of cell supernatant were dropped in a flat bottom 96-well plate, incubated with 50 μl of CytoTox-ONE™ reagent for 30 s on the microplate shaker and for 20 min in the dark at room temperature (RT). A stop solution (25 μl) was added to each well and the plate was placed on the microplate shaker for 10 s. Ultimately, the fluorescence was measured at an excitation wavelength of 560 nm and an emission wavelength of 590 nm using a CLARIOstar® microplate reader (BMG LABTECH).
Flow cytometry
Tumours were mechanically dissociated with scissors and digested enzymatically using the Tumour Dissociation Kit mouse and the gentleMACS Dissociator (Miltenyi Biotec) according to the manufacturer's instructions. After filtration through a 70 μm cell strainer (Miltenyi Biotec), the single-cell suspension was washed two times with complete RPMI-1640. Following the last centrifugation, the pellet was resuspended with a volume of complete RPMI-1640 to obtain a final concentration of 14 mg of tumour for 100 μL. Then, 100 μL of cell suspension was dropped in U 96-well plates for assessment of immune cell populations in the same amount of tumour for each one. Spleens and tumour draining lymph nodes (TDLN) were mechanically dissociated through 100 μm cell strainers (Falcon®). After red blood cell lysis, splenocytes were washed one time with complete RPMI-1640. 1 million of splenocytes and 0.5 million of cells from TDLN were used for staining in U 96-well plates. After PBS 1× washing, cells were stained with LIVE/DEAD™ Fixable Red Dead Cell Stain Kit for 30 min protected from light at RT. Fc receptors were blocked with anti-CD16/CD32 (TruStain FcX™; Biolegend) and cells were stained for cell surface antigens. For intracellular staining, cells were fixed and permeabilized using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience™). For ex vivo stimulation, intratumoral immune cells and splenocytes were restimulated with class II-restricted OVA-peptide (323–339) and/or class I-restricted OVA-peptide (257–264) for 4 h or 16 h respectively, both with PTI. Events were acquired on BD-LSRII flow cytometer (BD Biosciences, Le Pont-De-Claix, France), collected with BD FACSDiva 6.3.1 software and analysed using Flowlogic software (Miltenyi Biotec). All antibodies used for extracellular and intracellular stainings are listed in the Supplemental Table S4.
Immunohistochemistry
FFPE blocks were sectioned at 4 μm thickness using a microtome. Paraffin was removed and antigen retrieval was performed using a PT-Link apparatus (Agilent) at 97 °C for 20 min. After these steps, endogenous peroxidases were blocked for 5 min (Agilent, ref. SM801) and then the slides were incubated with CD8 antibody (Histosure, polyclonal, RRID: AB_2620121, 1/100, 30 min), CD4 antibody (Histosure, clone Rb78H9D2, RRID: AB_2884926, 1/900, 30 min) or PD1 antibody (Cell Signalling, clone D7D5W, RRID: AB_2800041, 1/50, 60 min). Slides were washed and then incubated with secondary HRP polymer reagents (Vector Laboratories, ref. MP7451) for 20 min. The slides were then washed and incubated with magenta chromogen (Agilent, ref. GV92511-2) for 5 min. After a final washing step, the slides were finally counterstained with haematoxylin (Agilent, ref. SM806) for 5 min. All staining steps were performed using an Autostainer AS48 (Agilent). Slides were digitised using a VS200 slide scanner (Evident) at ×20 magnification. Analysis of staining and quantification was performed using QuPath software (Bankhead, 2017, Sci reports).
Bulk RNA-sequencing from B16-OVA tumours
Tumours were snap frozen in liquid nitrogen and stored at −80 °C until use. RNA extraction, library preparation, RNA sequencing (RNA-seq), and determination of differentially expressed genes were performed by BGI genomics (Hong Kong, China). Total RNA was extracted from frozen B16-OVA tumours lysed by TissueLyser II (QIAGEN) using phenol-chloroform extraction method with Trizol (Invitrogen) and Isopropanol (XILONG). The quality of the total RNA and the sample RNA concentration were determined by using the Bioanalyzer 2100 (Agilent). 1 μg of total RNA was used to build mRNA libraries using MGIEasy RNA Library Prep kit (MGI, China) following the manufacturers' instructions. Briefly, mRNAs were first enriched using oligo (dT)-attached magnetic beads. Following mRNA fragmentation, first and second strand cDNA was synthesised using random primers (MGI, China). cDNA fragments were end-repaired, adenylated at 3′ ends, purified with magnetic beads by using a linker which was connected to the A base under enzymatic reaction, and enriched by PCR. After controlling the quality of PCR products on the Bioanalyzer 2100 (Agilent Technologies Inc.), double stranded PCR products were heat denatured and circularised by the splint oligo sequence. The single strand circle DNA (ssCir DNA) were formatted as the final library. The library was amplified to make DNA nanoball (DNB) which had more than 300 copies. The DNBs were load into the patterned nanoarray and sequenced on the DNBseq G400 (MGI, China) with read length as 2 × 150 bp paired end reads. Clean reads were then aligned against Mus musculus mm39 reference genome (source: UCSC) using HISAT. Analysis were performed with Dr. Tom online system and details are mentioned in the figure legends. Data are deposited in the Gene Expression Omnibus database under accession number GSE282402.
Statistics
Data were analysed with Prism software version 8.0.2 (Graph Pad software, La Jolla, California, USA) and shown as mean ± SEM. Data normality was tested with a D'Agostino and Pearson test. Mann–Whitney U test were used for comparisons of two groups. For multiple comparisons one-way or two-way ANOVA with Dunnett's or Tukey's multiple comparison post-hoc tests or Kruskal–Wallis test with Dunn's multiple comparisons test were performed when appropriate. For survival curve statistics, log-rank (Mantel–Cox) test was used. For correlation analysis, a Spearman r correlation coefficient was calculated. Statistical test used are specified in each figure legends. P < 0.05 was considered statistically significant.
Role of funders
The funders did not influence the study design, data collection, data analysis, interpretation, or the writing of this manuscript.
Results
Activation of DCs treated with NLC-Ova mRNA and ability of treated-DCs to specifically activate T cells in vitro
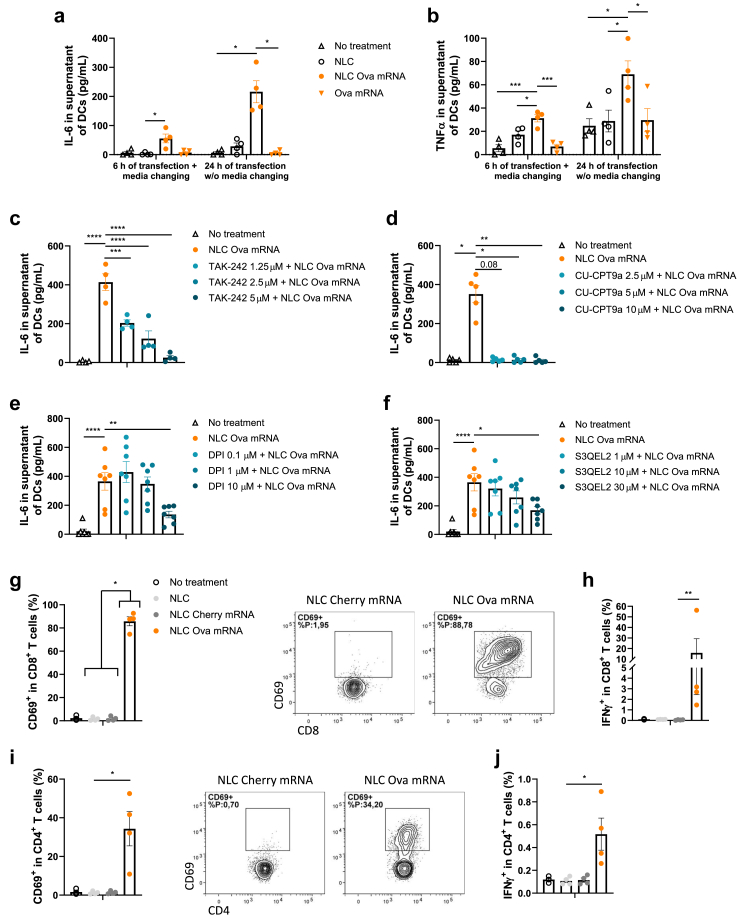
Given the crucial role of DCs as APCs in the antitumour effect of the mRNA-based vaccine, we tested whether our vaccinal strategy has an impact on DC activation in vitro. For this, we tested if Ova mRNA alone or vectorised affects the secretion of proinflammatory cytokines in BMDCs for a short and a long transfection timing. While NLC or Ova mRNA alone does not lead to IL-6 or TNFα secretion, Ova mRNA vectorised with NLC for 6 h and 24 h of transfection increases the concentrations of these two cytokines into the DC supernatant at day 1 (Fig. 1a and b). Previous studies reported that mRNA-based vaccines activate TLR pathways [24,25] and TLR activation increases ROS production in DCs [44]. We thus evaluated the contribution of TLR4, TLR8, and ROS signalling in mediating the proinflammatory effect of the vectorised Ova mRNA in BMDCs by using specific inhibitors. The inhibition of TLR4 and TLR8 by TAK-242 and CU-CPT9a, respectively, drives a drastic decrease in the NLC-Ova mRNA-mediated IL-6 release (Fig. 1c and d). Likewise, the inhibition of NOX-dependent ROS and mitochondrial ROS (mROS) by DPI and S3QEL2, respectively, reduces the IL-6 secretion in response to vectorised mRNA (Fig. 1e and f). Importantly, for all the doses, the TLR and ROS inhibitors used in combination with the NLC-Ova mRNA complex do not exhibit any effect on cell viability excluding cell death as a potential event leading to the lower IL-6 secretion (Supplemental Figure S1a and b).
Fig. 1.
NLC-Ova mRNA mediates BMDC and T cell activation in vitro. (a, b) IL-6 (a) and TNFα (b) concentration in BMDC supernatant at day 1 following a 6 h or a 24 h time incubation with vehicle, NLC, NLC-Ova mRNA or Ova mRNA. (c–f) IL-6 concentration in BMDC supernatant following 3 h of pretreatment with TLR4 inhibitor TAK-242 at three different concentrations (1.25 μM, 2.5 μM, and 5 μM) (c) or TLR8 inhibitor CU-CPT9a (2.5 μM, 5 μM, and 10 μM) (d) or following 1 h of pretreatment with ROS-dependent NOX inhibitor DPI (0.1 μM, 1 μM, and 10 μM) (e) or mitochondrial ROS (mROS) inhibitor S3QEL2 (1 μM, 10 μM, and 30 μM) (f) and 24 h of NLC-Ova mRNA incubation. Mean ± SEM of 4–7 independent experiments where each dot represents the mean of 2 replicates. (g–j) CD8+ T cells (g, h) or CD4+ T cells (i, j) from OT-I or OT-II spleen respectively were cocultured for 48 h with BMDCs treated with vehicle, NLC, NLC-cherry mRNA or NLC-Ova mRNA. Frequencies of CD69+ (g, i) and IFNγ+ (h, j) were assessed by flow cytometry. Mean ± SEM of four independent experiments where each dot represents the mean of 2–3 replicates. P values (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001) determined by Kruskal–Wallis test with Dunn's post hoc testing (a, d, g–j) or one-way ANOVA with Dunnett's post hoc testing (b, c, e, f).
To evaluate if BMDCs treated with NLC-Ova mRNA complex are able to translate mRNA, process antigens and present them to T cells, we cocultured them with CD8+ or CD4+ T cells isolated from spleen of OT-I or OT-II mice, respectively. The frequency of activated CD8+ lymphocytes (CD69+) is considerably increased (Fig. 1g), together with an enhanced production and secretion of IFNγ (Fig. 1h; Supplemental Figure S1c), TNFα (Supplemental Figure S1d and e), and IL-2 (Supplemental Figure S1f) compared with control conditions. Although to a lesser extent, similar results for CD69, IFNγ, and TNFα are observed in CD4+ T cells (Fig. 1i and j; Supplemental Figure S1g–i).
Altogether, these results indicate that NLC-Ova mRNA activates DCs in vitro in a TLR4/8 and ROS dependent manner, effectively delivers antigen-encoding mRNA to BMDCs and promotes efficient mRNA translation and antigen presentation for specific CD8+ and CD4+ T cell activation.
Antitumour efficacy of NLC-Ova mRNA-based vaccine in a cancer prevention setting
Because of the capacity of the NLC-Ova mRNA complex to lead to a cellular immune response in vitro we next evaluate whether vectorised Ova mRNA prevents tumour growth in vivo. Importantly, through in vivo biodistribution analysis, we validate an accumulation of the NLC-Ova mRNA vaccine in diverse lymph nodes with an increase of the signal between 5 and 24 h notably into the axillary lymph nodes (Supplemental Figure S2a and b). This targeting to lymph nodes is crucial for both the safety and efficacy of vaccinal strategy [45,46]. For other organs, vaccine distribution is greatest in the pancreas and liver (Supplemental Figure S2a and c) which is an expected result due to the i.p. injection route, a phenomenon already described by Melamed et al. [47]. In contrast to the results in the lymph nodes, we observe a decrease of the signal over time into the heart, the lung, the brain, the kidney, and the blood (Supplemental Figure S2a and c). Then, we immunised healthy adult mice three times with 3 μg of vectorised mRNA, grafted B16-OVA melanoma cells intradermally 6 days after the last immunisation and followed the tumour size evolution (Fig. 2a). In the NLC-Ova mRNA vaccine group, the tumour growth was completely impeded (6/6 mice) in comparison with the control group vaccinated with a mRNA encoding an irrelevant protein (Fig. 2b and c). Then, we assessed the activity of T lymphocytes at the periphery level since no tumours were available in the treated group for subsequent analysis. Under NLC-Ova mRNA treatment conditions, the frequency of CD8+ T cells from the spleen producing IL-2, IFNγ, GzmB or TNFα, coproducing IFNγ and TNFα and expressing PD-1 increases when compared to the control group (Fig. 2d–i) based on fluorescence minus one (FMO) controls for positive threshold determination (Supplemental Figure S3a). Similar changes in CD4+ T cells from the spleen, CD8+ and CD4+ T cells from the TDLN producing IL-2, IFNγ and TNFα were not detected (Supplemental Figure S3b–j). Moreover, we observed a higher concentration level of IFNγ in the splenocyte supernatant of the NLC-Ova mRNA group which would reflect the activation of the CD8+ rather than the CD4+ T cell, consistent with the flow cytometry results (Supplemental Figure S3k). Finally, we assessed whether the vaccine-induced antitumoral effect is still efficient even after a longer time (41 days) between the last vaccination and the tumour cell inoculation. Immunisation with NLC-Ova mRNA leads to complete protection upon tumour challenge also with this experimental setting (Supplemental Figure S3l and m). Overall, these results demonstrate that vectorised Ova mRNA can be used as an effective vaccine to prevent tumour development from OVA-expressing cancer cell engraftment together with the establishment of activatable and potentially mobilisable CD8+ T cells from the periphery.
Fig. 2.
Antitumour effect of NLC-Ova mRNA complex as a preventive vaccine. (a–c) Tumour growth in NLC-Ova mRNA immunised mice injected i.d. with B16-OVA melanoma cells. NLC-Cherry mRNA was used for the control group (b). Representative pictures of the B16-OVA tumour from NLC-Ova mRNA or NLC-Cherry mRNA treated mice at sacrifice (c). (d–i) CD8+ T cell analysis from spleen of B16-OVA tumour-bearing mice immunised with NLC-Ova mRNA or NLC-Cherry mRNA. Following ex vivo restimulation with OVA peptides, IL-2 (d), IFNγ (e), GzmB (f), TNFα (g) production, IFNγ and TNFα coproduction (h), and PD-1 expression (i) were assessed by flow cytometry. Mean ± SEM of N = 6 mice per group. P values (∗∗p < 0.01) determined by Mann–Whitney test (b–i).
Antitumour efficacy of NLC-Ova mRNA-based vaccine in a cancer therapeutic setting
We tested the therapeutic efficacy of our vaccinal strategy using two OVA-expressing murine tumour models. After B16-OVA or E.G7-OVA tumour cell inoculation, three immunisations with 3 μg of vectorised Ova mRNA were performed. In the groups treated with NLC-Ova mRNA, regardless of the type of tumour tested, tumour growth and weight at sacrifice are drastically reduced while spleen weight is not affected when compared to the control groups (Fig. 3a and b; Supplemental Figure S4a and b). Intratumoral T-cell specific immune response was assessed by flow cytometry after restimulation with OVA peptides for 4 h (for gating strategy see Supplemental Figure S4c) and immunohistochemistry (IHC). NLC-Ova mRNA-based vaccination increases the percentage of CD45+ cells in live cells, CD8+ T cells in CD45+ cells as well as the number of B16-OVA tumour-infiltrating CD45+, CD8+ and CD4+ cells compared with the NLC-irrelevant mRNA-treated mice (Fig. 3c–f; Supplemental Figure S4d–f). In addition, we noted higher frequencies of intratumoral activated CD8+ T cells expressing PD-1, CD69, producing IFNγ, TNFα or GzmB as well as coproducing IFNγ and TNFα or IFNγ and GzmB in the NLC-Ova mRNA group (Fig. 3g–j; Supplemental Figure S4g–j). Concerning the CD4+ T cells, only the frequencies of CD69+ and TNFα+ were significantly increased in this group (Supplemental Figure S4k–n). For the E.G7-OVA tumour model, similar results were observed. The LPN-Ova mRNA-based vaccination leads to a higher frequency of intratumoral CD8+ T cells in CD45+ cells as well as higher frequencies of CD8+ T cells expressing PD-1 and coproducing cytokines and GzmB when compared to the control group (Supplemental Figure S5a–d). Although the frequency of CD4+ T cells in CD45+ cells was not affected, the frequency of CD4+ T cells producing IFNγ and TNFα were increased in the NLC-Ova mRNA group (Supplemental Figure S5e–h). We also observe a negative correlation between percentage of activated CD8+CD69+ T cells and tumour weight at sacrifice which is more significant than with activated CD4+CD69+T cells (Fig. 3k). Because of the massive CD8+ T cell infiltration into the tumour, their clear enhanced functional profile and their correlation with the tumour weight, we completed our study by depleting CD8+ T in the B16-OVA tumour model. This experiment reveals that NLC-Ova mRNA-based vaccination efficacy is CD8+ T cell-dependent since the median survival of 34.5 days in this group was reduced to 21.5 days in the group vaccinated with NLC-Ova mRNA and blocked for CD8+ T cells, a value close to the control group (20.5 days) (Fig. 3l; Supplemental Figure S5i and j). Overall, these results indicate that vectorised Ova mRNA is an effective vaccine strategy for inhibiting tumour growth in a curative setting which mainly relies on enhanced CD8+ T cell effector function.
Fig. 3.
Antitumour effect of NLC-Ova mRNA complex as a therapeutic vaccine. (a, b) Tumour growth in mice injected with B16-OVA melanoma cells (a) or E.G7-OVA lymphoma cells (b) and then treated with NLC-Ova mRNA or NLC-irrelevant mRNA. (c–j) Intratumoral frequency of CD45+ cells in live cells (c), CD8+ T cells in CD45+ cells (d), number of CD8+ T cells per mg of B16-OVA tumour tissue (e) from NLC-Ova mRNA or NLC-Cherry mRNA-treated mice. Surface of CD8+ T cells (f) and PD-1+ T cells (g) analysed by IHC for each tumour group. Intratumoral frequency of CD8+ T cells expressing CD69 (h) and coexpressing IFNγ and TNFα (i) or IFNγ and GzmB (j) from B16-OVA tumour-bearing mice treated with NLC-Ova mRNA or NLC-irrelevant mRNA following OVA peptide stimulation. (k) Correlations between intratumoral CD69+CD8+ T cells frequency or CD69+CD4+ T cells frequency and tumour weight at day 17 in B16-OVA-bearing tumour mice treated with NLC-Ova mRNA or NLC-Cherry mRNA. (l) Survival in B16-OVA tumour-bearing mice treated i.p. with NLC-Egfp mRNA or NLC-Ova mRNA as well as with anti-CD8β or its control IgG1. Mean ± SEM of N = 6 mice per group. P values (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001) determined by Mann–Whitney test (a–j) or Spearman r correlation test (k) or log-rank (Mantel–Cox) test (l).
NLC-Ova mRNA-based vaccine reprogrammes the intratumoral immune-related transcriptional signature in a favourable way to anti-PD-1 response
An unbiased RNA sequencing (RNA-seq) was performed following two immunisations at day 9 to evaluate early changes in gene expression induced by NLC-Ova mRNA-based vaccine in B16-OVA tumour bearing mice (Supplemental Figure S6a). The results reveal the upregulation of many genes involved in the immune response as shown in the volcano plot, especially genes related with tumoricidal effector T cells (Ifng, Gzma, Gzmb, Cd8a, Cd69) and lymphocyte attraction (Cxcl10, Cxcl11, Cxcl9) (Fig. 4a). Overall, 487 genes were differentially expressed after vaccine treatment (defined as a log2 fold change >2 and Q value < 0.005) (Supplemental Figure S6b). Gene Set Enrichment Analysis (GSEA) based on these 487 genes shows that NLC-Ova mRNA treatment leads to a selective enrichment of biological pathways involving notably the “cellular response to IFN-beta” and “cellular response to IFN-gamma” (Fig. 4b–d). Then, a KEGG pathway classification was realised and the higher number of genes modified (123) in response to the NLC-Ova mRNA-based vaccine referred to the “immune system” pathway (Fig. 4e). Based on these 123 genes, we performed a KEGG pathway relationship network (Supplemental Figure S6c) and selected, ranked by the number of genes and p value, the three most significative pathways to generate gene expression heatmaps which are related to the “T cell receptor signaling pathway”, “chemokine signaling pathway”, and “antigen processing and presentation” (Fig. 4f and g; Supplemental Figure S6d, respectively). Importantly, our vaccinal strategy converted the cold intratumoral gene signature to an immune-related signature correlating with clinical benefit to PD-1 blockade in patients with melanoma [8] and potentially predicting the synergetic action of this therapeutic combination (Supplemental Figure S6e). Each gene of all the explored pathways here shows a significant upregulation in the NLC-Ova mRNA treated group (log2 fold change >2 and Q value < 0.005). Altogether, these proinflammatory gene expressions cooperate to build up an immunoreactive tumour microenvironment that favoured CD8+ T cell accumulation and activation in a favourable way to anti-PD-1 response.
Fig. 4.
NLC-Ova mRNA vaccine produces an inflamed immune gene signature. Mice bearing B16-OVA tumours were treated with NLC-Egfp mRNA or LNP-Ova mRNA twice and analysed by RNA-seq. (a) Volcano plot showing the fold change (log2, x-axis) and statistical significance (−log10 Q value, y-axis) of differentially expressed genes (DEGs) (log2 fold change >2 and Q value < 0.005). (b) Top 10 of selective enrichment of biological pathways following GSEA based on 487 DEGs between NLC-Egfp mRNA and NLC-Ova mRNA groups (log2 fold change >2 and Q value < 0.005). (c, d) Enrichment plot of indicated signatures. Normalised Enrichment score (NES), False Discovery Rate (FDR) Q value shown for the two gene sets. (e) KEGG pathway classification based on 487 DEGs between NLC-Egfp mRNA and NLC-Ova mRNA groups (log2 fold change >2 and Q value < 0.005). (f, g) Heatmaps of genes related to “T cell receptor signaling pathway” (f) and “chemokine signaling pathway” (g) following the selection of the most significative pathways from a KEGG pathway relationship network based on the 123 genes related to the “immune system” pathway. N = 4 mice per group.
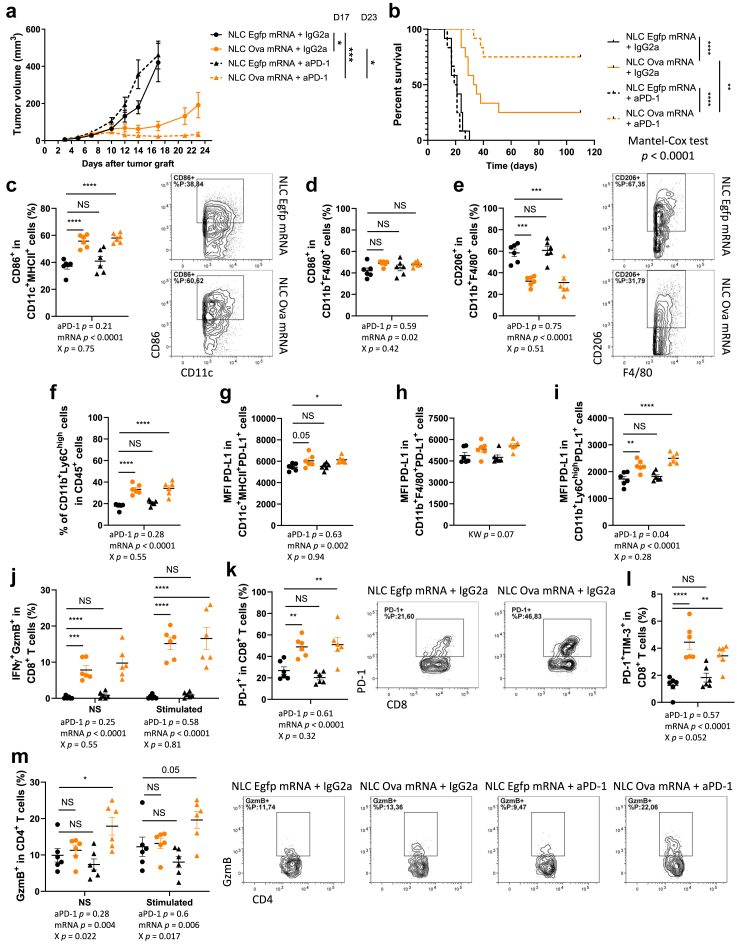
Anti-PD-1 blocking antibody synergises with anticancer effect of NLC-Ova mRNA-based vaccine
Given that our vaccine strategy renders the immunologically inactive and anti-PD-1-resistant B16-OVA tumour model immunologically active, we tested whether NLC-Ova mRNA treatment would result in sensitisation of this model to anti-PD-1 immunotherapy. Furthermore, in addition to an increased frequency of CD8+ T cells expressing PD-1 in B16-OVA and E.G7-OVA tumour models in response to the NLC-Ova mRNA treatment (Fig. 3g; Supplemental Figure S5b), we observed a greater fraction of inflammatory monocytes that expresses PD-L1 in the E.G7-OVA lymphoma model (Supplemental Figure S7a–c) as well as a higher gene expression of Cd274 and Pdcd1 in B16-OVA tumour (Fig. 4a–f). These results suggest that the PD-1/PD-L1 axis can be a possible mechanism of immune escape in response to long-term vaccine-induced antitumour action. To test this, we immunised mice with NLC-Ova mRNA and treated them with anti-PD-1 using the B16-OVA tumour model (for treatment schedule see Supplemental Figure S7d). In these experiments we still show the vaccine-mediated antitumour effect, and, we observe that this effect is transient. Indeed, the resumption of tumour growth appears in majority of mice; only 3/12 mice survive after 100 days following tumour cell injection in the NLC-Ova mRNA-treated group (Fig. 5a and b; Supplemental Figure S7e), whereas the combination with anti-PD-1 considerably improves the tumour growth control and the mouse survival since 9/12 mice are still alive at day 100 in the group treated with Ova mRNA-based vaccine and anti-PD-1 (Fig. 5a and b; Supplemental Figure S7e). In order to better understand the potential early immune-related mechanisms underlying the beneficial effect of this combinatorial treatment, we analysed the intratumoral myeloid, T, and NK cells at day 9, two days after the first injection of anti-PD-1. At this timing we mainly observed a vaccine effect on myeloid cells leading to a higher intratumoral frequency of activated DCs, activated macrophages, inflammatory monocytes (p < 0.0001, p = 0.02, p < 0.0001 respectively) and a reduced fraction of M2 CD206+ macrophages (p < 0.0001) (Fig. 5c–f; for gating strategy see Supplemental Figure S8). While a trend was notable for macrophages, DCs and inflammatory monocytes show a statistically significant more intensive expression of PD-L1 in NLC-Ova mRNA treated mice especially in the group combined with anti-PD-1 (Fig. 5g–i). Interestingly, while the vaccine treatment raises the fraction of CD8+ T cells expressing GzmB, IFNγ, and PD-1 (Fig. 5j and k; for gating strategy see Supplemental Figure S9a), we observe a close statistically significative interaction between aPD-1 and vaccine therapies leading to a partial prevention of PD-1+TIM-3+ CD8+ T cell accumulation and a statistically significative interaction leading to a higher proportion of CD4+ T producing GzmB in the group treated with the treatment combination (Fig. 5l and m). The IFNγ serum concentration is also higher in the groups treated with the vaccine in comparison to the NLC-Egpf mRNA + IgG2a group more probably related to the CD8+ T cell-mediated IFNγ secretion since the production of CD4+ T cells was not modulated by any treatment at this time point (Supplemental Figure S9b and c). Last, although the NLC-Ova mRNA treatment enhances the intratumoral frequency of NK cells and their production of GzmB, the blocking of these cells does not impair the vaccine-mediated antitumoral efficacy while it shortens the survival of mice treated with NLC-Egpf mRNA (Supplemental Figure S9d–f). Altogether, these results highlight the emergence of a PD-1 dependent immune escape process in response to the vaccine, which can be blocked to improve survival in mice. At this early stage, the combination of the vaccine and anti-PD-1 therapy particularly targets CD4+ T cells by enhancing their cytotoxic function.
Fig. 5.
NLC-Ova mRNA vaccine cooperates with anti-PD-1 to suppress B16-OVA tumour growth. (a, b) Tumour growth (a) and survival (b) in mice injected with B16-OVA tumour cells and treated i.p. with NLC-Ova mRNA or NLC-Egfp mRNA as well as with anti-PD-1 or its control IgG2a. Mean ± SEM of N = 12 mice per group. P values (∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001) determined by Kruskal–Wallis test with Dunn's post hoc testing (a, D17) and Mann–Whitney test (a, D23) or log-rank (Mantel–Cox) test (b). (c–m) Intratumoral frequency of CD86+ DCs (CD11c+MHCII+) (c), CD86+ macrophages (CD11b+F4/80+) (d), CD206+ macrophages (e) and proinflammatory monocytes (CD11b+Ly6Chigh) (f) PD-L1 expression (MFI) in PD-L1+ DCs (g), PD-L1+ macrophages (h) and PD-L1+ inflammatory monocytes (CD11b+Ly6Chigh) (i); intratumoral frequency of CD8+ T cells coexpressing IFNγ and GzmB (j), expressing PD-1 (k), coexpressing PD-1 and TIM-3 (l) and intratumoral frequency of CD4+ T cells expressing GzmB (m) from B16-OVA tumour-bearing mice treated with NLC-Ova mRNA or NLC-Egfp mRNA as well as with anti-PD-1 or IgG2a. Mean ± SEM of N = 6 mice per group. P values (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001) determined by two-way ANOVA (mRNA vaccine effect (mRNA) anti-PD-1 treatment effect (aPD-1) and their interaction (X)) with Dunnett's post hoc testing (c–g, i–m) or Kruskal–Wallis test with Dunn's post hoc testing (h). NS, not significant.
Long-term memory immune response in cancer survivor mice
In order to evaluate the memory antitumoral immune response and its ability to prevent the development of a new tumour, the surviving mice treated with vectorised Ova mRNA or the combination vectorised Ova mRNA and anti-PD-1 were challenged with a second injection of B16-OVA at 103 days. Tumour growth in the challenged mice is largely blocked; the size of the tumours at day 5 of follow-up is already significantly lower than the values of the control group (Fig. 6a). Since the tumours were too small for analysing the intratumoral immune response, we evaluated different immune cell populations in the spleen. Following an ex vivo restimulation, the production of IFNγ, TNFα or both (frequencies and MFI) by CD8+ splenocytes is higher in surviving mice than in animals of the control group (Fig. 6b–f). Similarly, Ova-specific CD8+ T cells (Ova dextramer) are more frequent in the spleens of challenged mice (Fig. 6g). Moreover, although the frequencies of DCs and B cells are not modified in CD45+ cells (Supplemental Figure S10a–c), their level of activation is higher (↑ MFI of CD86 in CD86+ DCs and ↑ frequency of CD86+MHCII+ B cells) in the spleen of treated mice having survived their 1st tumour without PD-L1 expression changes (Fig. 6h and i; Supplemental Figure S10e–g). Finally, we challenged another batch of surviving mice with B16 cells in order to assess if they developed an antitumour memory immune response against antigens other than those derived from OVA. Although the tumour growth in the challenge group was not completely stopped as we observed with OVA-expressing B16 melanoma cells, the tumour size from day 4 until day 14 as well as the tumour weight at sacrifice were lower than in the control group (Fig. 6j and k). While the frequency of intratumoral activated CD8+ T cells was not different between these two groups (Supplemental Figure S10h and i), the frequency of activated NK cells was increased in the challenge group and negatively correlated with the final tumour weight (Fig. 6l and m). Altogether these results demonstrate that in responsive mice to our vaccinal strategy in combination or not with anti-PD-1 a long-lasting memory antitumoral immune response was induced in association with the peripheral establishment of polyfunctional and antigen-specific CD8+ T cells and activated APCs without PD-L1 expression-related immunosuppression. This memory immune response is not restricted to OVA-derived antigens suggesting that treatment-related antigen spreading occurs.
Fig. 6.
Protection from tumour re-challenge in survivor mice treated with NLC-Ova mRNA ± anti-PD-1. (a) Tumour growth in NLC-Ova mRNA- (orange dot surrounded by a black circle) or NLC-Ova mRNA + anti-PD-1- (orange dot) treated surviving mice challenged with a second injection of B16-OVA cancer cells 103 days following the first B16-OVA tumour cell injection. Control mice (black dot) were similar age than challenged mice and were injected with the same number of B16-OVA tumour cells (2 × 105). (b–i) Frequency of CD8+ T cells expressing IFNγ (b, c), TNFα (d, e), coexpressing IFNγ and TNFα (f); frequency of OVA-specific CD8+ T cell (g); expression level of CD86 (MFI) in CD86+ DCs (h) and frequency of B cells expressing CD86 and MHC-II (i) into the spleens from control and challenged B16-OVA tumour-bearing mice following OVA peptide stimulation. (j, k) Tumour growth (j) and weight (k) in NLC-Ova mRNA- or NLC-Ova mRNA + anti-PD-1-treated surviving mice challenged with an injection of B16 cancer cells 113 days following the first B16-OVA tumour cell injection. (l, m) Intratumoral frequency of NK cells expressing CD69 from control and challenged B16 tumour-bearing mice (l) as well as correlation between B16 intratumoral frequency of CD69+ NK cells and tumour weight at day 14 (m). Mean ± SEM of N = 9 mice per group (a–g) or N = 5–6 mice per group (h–m). P values (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001) determined by Mann–Whitney test (a–l) or Spearman r correlation test (m). NS, not significant.
Discussion
Therapeutic cancer vaccine is a potential strategy to convert poorly immunologic tumours resistant to anti-PD-1 into inflamed immune tumoral microenvironments favourable for anti-PD-1 efficacy. Here, we demonstrated that mRNA-based vaccine activates DCs in vitro in TLR and ROS-dependent signalling pathways and prevents tumour growth in a CD8+ T cell-dependent manner in association with an intratumoral IFNγ and antigen presentation gene signature previously shown to correlate with responsiveness to PD-1 blockade in patients with melanoma [8,42]. We also found that the blockade of PD-1 in vaccinated mice improves the early production of GzmB by intratumoral CD4+ T cells, the survival and the rate of complete tumour regression. Importantly, we observed resistance to tumour growth in surviving mice reexposed to cancer cells expressing or not OVA illustrating immunologic memory establishment and suggesting antigen spreading.
DCs are main players in initiating the LNP-mRNA vaccine-induced antitumoral immune response thanks to their ability to detect pathogen-associated molecular patterns (PAMPs), notably nucleic acids and lipids, which activate TLRs leading to the triggering of pro-inflammatory reactions and the subsequent shaping of T cell differentiation [48,49]. We should recall that membrane/endosome-localised TLR4 has not been found involved in the mRNA-based vaccine response consensually [24,25] and the functionality of the endosome-localised TLR8 has been described to be controversial in mice [50]. Here, we observed a TLR4-and TRL8-dependent DC activation following exposure to NLC-Ova mRNA. This is probably linked to the cationic lipid composition of the Lipidots® and the ssRNA form of mRNA, respectively [49]. It has been reported that, TLR agonists such as R848 (TLR7/8) or LPS (TLR4) can trigger ROS-dependent activation and maturation of DCs [44] and that ROS production in DCs plays a crucial role for the induction of CD8+ T cell responses following in vivo immunisation [44] as well as for the proliferation of CD4+ T cells following in vitro TLR activation [51]. Consistent with these studies, we found that DC exposition to NLC-Ova mRNA leads to a NADPH oxidase and mitochondria derivative ROS-dependent activation besides generating OVA-specific CD4+ and CD8+ T cell activations. Altogether this highlights the potential crosstalk between TLR and ROS signalling pathways in DC response to mRNA-based vaccine leading to the downstream activation of the adaptative immunity.
In this study we used an unmodified mRNA due to its ability to induce signalling by type I IFN which is necessary for the induction of a protective CD8+ T cell response [33]. Compared with modified mRNA encoding OVA, unmodified mRNA can induce higher frequencies of GzmB+/IFNγ+/TNFα+ polyfunctional OVA peptide-specific CD8+ T cells into the spleen and intratumoral activated DCs in association with a robust antitumoral effect as reported in the study of Sittplangkoon et al. [33]. However, in another study, even if unmodified mRNA leads to higher level of circulating OVA-specific CD8+ T cells, better shrinkage of tumour growth is not observed in comparison to a modified mRNA [26]. We have shown previously [40] and confirm here that the NLCs we use in this study do not activate DCs solely by themselves unlike LNPs showing intrinsic adjuvant activity. Although modified mRNA improves the translation into protein antigen, its complexation with Lipidots® could require the addition of an adjuvant to present a complete vaccinal strategy with optimal antitumoral impact.
Here we report that, besides having in vivo prophylactic efficacy in association with the generation of splenic polyfunctional CD8+ T cells, NLC-Ova mRNA vaccine displays a CD8+ T cell-dependent antitumoral therapeutic efficacy through the robust intratumoral infiltration of OVA-specific polyfunctional CD8+ T cells. Our results are consistent with previous preclinical studies showing that mRNA-based vaccines delivering mRNA coding for OVA or tumour antigens (e.g., GP70, TRP-1, TRP-2, MART1, MUC1, E6 or E7, etc.) reduce tumour burden [23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33]. Although some of them demonstrate a CD8+ T cell infiltration into the tumour [30, 31, 32, 33], for the most part, the functionality of CD8+ T cells (production/secretion of IFNγ and/or TNFα and/or GzmB) was rather assessed at the splenic or lymph node level [24,25,28, 29, 30,32,33] than at the tumour level [23,27]. Beyond the implication of CD8+ T cells in mRNA vaccine-induced immunity, the intratumoral infiltration of NK cells has been reported with the use of an unmodified mRNA-LNP vaccine in B16-OVA tumour-bearing mice [31] as well as in other cancer models submitted to mRNA vaccine [23]. In addition to observe a higher GzmB production in NK cells at an early time point (day 9), we also find an increase of their intratumoral frequency, however, their blockade does not impair the vaccinal therapeutic efficacy in our conditions. The early response of NK cells to Ova mRNA vaccine may be not sustained over time to produce a dependent antitumoral effect because of their dysregulation within the TME as recently proposed [52].
Intratumoral cell population immunophenotyping and transcriptional analyses reveal that our mRNA vaccinal strategy promotes a proinflammatory TEM favourable to the transient control of tumour growth while inducing escape mechanisms involving in particular the PD-1/PD-L1 signalling pathway. Blocking PD-1 in addition to the vaccination considerably improves the survival and the rate of complete tumour regression without, at the early time point assessed, deeply impacting the myeloid cell compartment which is mainly affected by the mRNA vaccine. In line with our in vitro data and past preclinical works [23,24,33], NLC-Ova mRNA increases the frequency of mature DCs expressing CD86 while inducing a transcriptional type I IFN signature into the tumour. Type I IFN signalling pathway was shown to support the cross-priming of antigen-specific CD8+ T cells following DC activation by engagement of TLR such as TLR4 and TLR7/8 [53]. The involvement of this mechanism in the antitumour efficacy of NLC-Ova mRNA, although not directly assessed in vivo, would be coherent especially in regards to the in vitro vaccine-caused TLR-dependent DC activation we observe. Furthermore, NLC-Ova mRNA immunisation results in an early and robust reduction in the frequency of immunosuppressive CD206+ M2-like tumour-associated macrophages (TAMs) whereas that of inflammatory CD86+ M1-like TAMs is barely increased. Although these cells have been rarely explored intratumorally in response to anticancer mRNA vaccines [23,32], a similar phenotype skewing of macrophages has been found into the tumours of mice vaccinated with mRNA coding E7 [23]. Mechanistically, this phenotype commitment can be related to the IFNγ and/or TNFα signals through the massive intratumoral infiltration of IFNγ and TNFα producing CD8+ T cells following vaccination as already reported in a peptide-vaccine study [33,54]. Moreover, the antitumour efficacy of transferred antigen-specific CD8+ T cells can be promoted by the migration of Ly6Chigh monocytes into the tumour [55]. Interestingly, we also observe an infiltration of inflammatory monocytes in response to NLC-Ova mRNA vaccine concomitantly with better CD8+ T cell activity. However, these cells have a particularly high expression of PD-L1 following vaccination suggesting their potential dual role and involvement in the induction of a later CD8+ T cell dysfunction related to the resumption of tumour growth in the majority of animals treated only with the mRNA vaccine. Finally, the mRNA vaccine-induced upregulation of PD-L1 on proinflammatory monocytes, but also on DCs, probably linked to the high intratumoral levels of IFNγ, can be considered as an adjuvant strategy to the PD-1/PD-L1 blockade to both sustain an elevated T cell activation while preventing their mutual inhibition highlighting the interest in therapeutic combinations rather than monotherapy once again.
Although CD8+ T cells are undoubtedly crucial players in the anti-tumour response during treatment by immunotherapy in general and therapeutic vaccination in particular, the role of CD4+ T cells is increasingly investigated [56]. Usually, CD4+ T cells have been depicted as helper cells, furthermore, results from preclinical and clinical studies reveal their intrinsic cytotoxic properties as well as their capacity to kill directly cancer cells in a MHC class-II context through notably their production of GzmB [57,58]. In a new way compared to previous preclinical studies evaluating combinatory approaches [23,27], we observe at an early stage a positive interactive effect between mRNA vaccine and anti-PD-1 treatment on the intratumoral frequency of Gzmb+ CD4+ T cells suggesting the potential involvement of these cytotoxic cells in the effective control of the tumour progression in response to the combinatory therapy. These results are in line with the better clinical response to the PD-1/PD-L1 axis blockage associated with the presence of cytotoxic CD4+ T into the tumour [59]. Concerning the CD8+ T cells, although their ability to produce high levels of IFNγ and GzmB is still observed in vaccinated mice over time (day 9 and day 17), the frequency of PD-1+ CD8+ T cells is already doubled on day 9, reinforcing the interest of adding an anti-PD-1 therapy to maintain the initial antitumour effect of the vaccination-related massive infiltration of active cytotoxic CD8+ T cells.
Finally, another key observation is that our vaccinal strategy associated with an anti-PD-1 therapy mediates a strong and sustainable OVA-specific CD8+ T cell memory preventing the growth of a new OVA-expressing tumour. The antitumoral immune memory is rarely evaluated in the preclinical studies quoted above, however, in two studies mRNA vaccination targeting CT26-M90 neoepitope or E7 oncoprotein eradicates the initial tumour in 60% or 100% of cases respectively and protects mice from re-challenge. In our experiments, although survival mice treated with the vaccine alone are able to mount a memory immune response, the rate of initial complete tumour regression following vaccination is much lower (25%). These differences may be related to the modifications made or not to the mRNA, the adjuvancity of the lipid nanocarrier as well as the dose which is largely lower in our study when compared to the two others (3 μg here vs. 40 μg). Nevertheless, in most clinical trials therapeutic mRNA cancer vaccines are more likely to perform well in combination with other immunotherapeutic therapies [22,60,61]. We also demonstrate that our vaccine strategy sensitises an anti-PD-1 resistant tumour model to ICB thus supporting the interest in continuing to test these types of combination in patients.
Importantly, as shown with the B16 re-challenge experiment, a secondary immune response against non-OVA derivative antigens (antigen spreading) is fostered following initial therapy-mediated tumour destruction which is a required phenomenon for eliciting a durable immune response and improved outcomes in patients undergoing cancer immunotherapy [62,63]. While a higher intratumoral frequency of CD69+ CD8+ T cells in association with the decrease of B16 tumour growth in challenged mice was expected, we rather observe a higher frequency of activated NK cells which correlates negatively with the tumour weight. However, since the CD8+ T cell production of cytotoxic molecules (IFNγ, GzmB, etc.) has not been evaluated, the involvement of its improvement at this timing or at an earlier time remains possible. Furthermore, the concept of innate immune memory is less well characterised than adaptive immune memory, nevertheless, diverse subtypes of memory NK cells have been described according to the initial activation trigger [64]. While the induction of this memory phenotype has been demonstrated by a combination of cytokines in vitro with proven antitumour activity in vivo [65] as well as in response to viral infection [66], potential induction by immunotherapy has only been exceptionally investigated. To the best of our knowledge, a single paper demonstrates in a new way the role of memory-like NK cells in preventing melanoma lung metastasis through the release of cytokines induced by a combination of STING-LNPs and CpG-ODNs [67]. Our results also support this concept and highlight the potential importance of such cells in the prevention of cancer recurrence following combinatory immunotherapy.
To conclude, we demonstrate the capacity of Lipidots® to deliver mRNA into DCs in vitro and activate them through TLR and ROS signalling pathways. This easy-to-use Lipidots®, relying on their simple mixture with mRNA, is an attractive platform for developing mRNA-based vaccines allowing antitumoral immune responses. Our in-depth intratumoral investigations of a cold tumour model reveal some particular cellular mechanisms involved in the mRNA vaccine effectiveness such as the massive infiltration of polyfunctional CD8+ T cells and proinflammatory monocytes as well as the early DC activation and protumor M2 reduction. However, a mRNA vaccine-induced PD-1/PD-L1 escape mechanism appears which is effectively targetable with an anti-PD-1 therapy to foster complete tumour rejection, durable immune memory and antigen spreading. Finally, our results highlight the interest in deepening the role of cytotoxic CD4+ T lymphocytes and NK cells in future studies to optimise the initial and memory antitumoral immune responses of combinatory therapeutic strategies involving mRNA vaccines and ICBs.
Contributors
C. Fournier: conceptualisation, methodology, validation, formal analysis, investigation, writing–original draft, writing–review and editing, visualisation, supervision, project administration. M. Mercey-Rejessac: investigation, writing–review and editing. V. Derangère: validation, investigation, resources, visualisation. A. Al Kadi: investigation, visualisation. D. Rageot: investigation. C. Charrat: investigation. A. Leroy: investigation. J. Vollaire: investigation, visualisation. V. Josserand: investigation. M. Escudé: resources. S. Escaich: resources. F. Ghiringhelli: investigation, resources, provided critical feedback. T. Decaens: provided relevant comments. F. P. Navarro: resources, funding acquisition, writing–review and editing. E. Jouvin-Marche: funding acquisition, writing–review and editing. P.N. Marche: validation, resources, project administration, funding acquisition. V. Derangère, V. Josserand, F. P. Navarro, and P.N. Marche have accessed and verified the underlying data.
All authors read and approved the final version of the manuscript.
Data sharing statement
All relevant data supporting the findings of this study are available within the article or from the corresponding authors upon request.
Declaration of interests
T.D. declares consulting fees (BMS, AstraZeneca, Roche, BD), honoraria for lecture and presentations (BMS, Abbvie, Gilead, AstraZeneca, Roche), payment for expert testimony (HAS) and support for attending meeting (MSD, Abbvie, Gilead, AstraZeneca, Roche). The other authors declare that they have no competing interests.
Acknowledgements
We thank the Microcell core facility of the Institute for Advanced Biosciences (UGA - Inserm U1209 - CNRS 5309), especially Mylène Pezet, for her assistance with equipment. This facility belongs to the IBISA-ISdV platform, member of the national infrastructure France-BioImaging supported by the French National Research Agency (ANR-10-INBS-04).
We thank Emilie Rustique (CEA, LETI, Grenoble) for generating the NLC-IR780 used for the in vivo biodistribution and Marie-Line Cosnier for its help in the project tracking for CEA, LETI, Grenoble.
The Optimal small animal imaging platform is supported by France Life Imaging (French program “Investissement d'Avenir” grant; “Infrastructure d'avenir en Biologie Santé”, ANR-11-INBS-0006) and the IBISA French consortium “Infrastructures en Biologie Santé et Agronomie”.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105543.
Appendix ASupplementary data
References
- 1.Vinay D.S., Ryan E.P., Pawelec G., et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35(Suppl):S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Reck M., Rodríguez-Abreu D., Robinson A.G., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 3.Schachter J., Ribas A., Long G.V., et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390(10105):1853–1862. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 4.El-Khoueiry A.B., Sangro B., Yau T., et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rini B.I., Powles T., Atkins M.B., et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404–2415. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbst R.S., Soria J.C., Kowanetz M., et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayers M., Lunceford J., Nebozhyn M., et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y.T., Sun Z.J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11(11):5365–5386. doi: 10.7150/thno.58390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spranger S., Bao R., Gajewski T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz de Galarreta M., Bresnahan E., Molina-Sánchez P., et al. β-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9(8):1124–1141. doi: 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy P.M., Valdera F.A., Smolinsky T.R., et al. Tumor infiltrating lymphocytes as an endpoint in cancer vaccine trials. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1090533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan S., Fan R., Han B., Tong A., Guo G. The potential of mRNA vaccines in cancer nanomedicine and immunotherapy. Trends Immunol. 2024;45(1):20–31. doi: 10.1016/j.it.2023.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Weber J.S., Yang J.C., Atkins M.B., Disis M.L. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33(18):2092–2099. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenblum H.G., Gee J., Liu R., et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect Dis. 2022;22(6):802–812. doi: 10.1016/S1473-3099(22)00054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Sahly H.M., Baden L.R., Essink B., et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385(19):1774–1785. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Wang M., Peng X., et al. mRNA vaccine in cancer therapy: current advance and future outlook. Clin Transl Med. 2023;13(8) doi: 10.1002/ctm2.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan S., Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017;24(3):133–143. doi: 10.1038/gt.2017.5. [DOI] [PubMed] [Google Scholar]
- 19.Liang F., Lindgren G., Lin A., et al. Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in rhesus macaques. Mol Ther. 2017;25(12):2635–2647. doi: 10.1016/j.ymthe.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J., Ye Z., Huang C., et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc Natl Acad Sci U S A. 2022;119(34) doi: 10.1073/pnas.2207841119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahin U., Derhovanessian E., Miller M., et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 22.Rojas L.A., Sethna Z., Soares K.C., et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature. 2023;618(7963):144–150. doi: 10.1038/s41586-023-06063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grunwitz C., Salomon N., Vascotto F., et al. HPV16 RNA-LPX vaccine mediates complete regression of aggressively growing HPV-positive mouse tumors and establishes protective T cell memory. Oncoimmunology. 2019;8(9) doi: 10.1080/2162402X.2019.1629259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kranz L.M., Diken M., Haas H., et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H., You X., Wang X., et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc Natl Acad Sci U S A. 2021;118(6) doi: 10.1073/pnas.2005191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberli M.A., Reichmuth A.M., Dorkin J.R., et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17(3):1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bialkowski L., van Weijnen A., Van der Jeught K., et al. Intralymphatic mRNA vaccine induces CD8 T-cell responses that inhibit the growth of mucosally located tumours. Sci Rep. 2016;6 doi: 10.1038/srep22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Zhang L., Xu Z., Miao L., Huang L. mRNA vaccine with antigen-specific checkpoint blockade induces an enhanced immune response against established melanoma. Mol Ther. 2018;26(2):420–434. doi: 10.1016/j.ymthe.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Moignic A., Malard V., Benvegnu T., et al. Preclinical evaluation of mRNA trimannosylated lipopolyplexes as therapeutic cancer vaccines targeting dendritic cells. J Control Release. 2018;278:110–121. doi: 10.1016/j.jconrel.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 30.Liu L., Wang Y., Miao L., et al. Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Mol Ther. 2018;26(1):45–55. doi: 10.1016/j.ymthe.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verbeke R., Lentacker I., Breckpot K., et al. Broadening the message: a nanovaccine co-loaded with messenger RNA and α-GalCer induces antitumor immunity through conventional and natural killer T cells. ACS Nano. 2019;13(2):1655–1669. doi: 10.1021/acsnano.8b07660. [DOI] [PubMed] [Google Scholar]
- 32.Shi L., Yang J., Nie Y., Huang Y., Gu H. Hybrid mRNA nano vaccine potentiates antigenic peptide presentation and dendritic cell maturation for effective cancer vaccine therapy and enhances response to immune checkpoint blockade. Adv Healthc Mater. 2023;12(32) doi: 10.1002/adhm.202301261. [DOI] [PubMed] [Google Scholar]
- 33.Sittplangkoon C., Alameh M.G., Weissman D., et al. mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.983000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delmas T., Piraux H., Couffin A.C., et al. How to prepare and stabilize very small nanoemulsions. Langmuir. 2011;27(5):1683–1692. doi: 10.1021/la104221q. [DOI] [PubMed] [Google Scholar]
- 35.Delmas T., Couffin A.C., Bayle P.A., et al. Preparation and characterization of highly stable lipid nanoparticles with amorphous core of tuneable viscosity. J Colloid Interface Sci. 2011;360(2):471–481. doi: 10.1016/j.jcis.2011.04.080. [DOI] [PubMed] [Google Scholar]
- 36.Courant T., Bayon E., Reynaud-Dougier H.L., et al. Tailoring nanostructured lipid carriers for the delivery of protein antigens: physicochemical properties versus immunogenicity studies. Biomaterials. 2017;136:29–42. doi: 10.1016/j.biomaterials.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Bayon E., Morlieras J., Dereuddre-Bosquet N., et al. Overcoming immunogenicity issues of HIV p24 antigen by the use of innovative nanostructured lipid carriers as delivery systems: evidences in mice and non-human primates. NPJ Vaccines. 2018;3(1):46. doi: 10.1038/s41541-018-0086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarro F.P., Berger M., Guillermet S., et al. Lipid nanoparticle vectorization of indocyanine green improves fluorescence imaging for tumor diagnosis and lymph node resection. J Biomed Nanotechnol. 2012;8(5):730–741. doi: 10.1166/jbn.2012.1430. [DOI] [PubMed] [Google Scholar]
- 39.Bastogne T., Hassler L., Bruniaux J., et al. A bayesian implementation of quality-by-design for the development of cationic nano-lipid for siRNA transfection. IEEE Trans Nanobioscience. 2023;22(3):455–466. doi: 10.1109/TNB.2022.3213412. [DOI] [PubMed] [Google Scholar]
- 40.Dey A.K., Nougarède A., Clément F., et al. Tuning the immunostimulation properties of cationic lipid nanocarriers for nucleic acid delivery. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.722411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navarro F.P., Bruniaux J., Gidrol X., Sulpice E., Texier I. 2014. Formulation for the Delivery of Nucleotide Sequences that can Modulate Endogenous Interfering mRNA Mechanisms. WO2014032953A1. [Google Scholar]
- 42.Grasso C.S., Tsoi J., Onyshchenko M., et al. Conserved interferon-γ signaling drives clinical response to immune checkpoint blockade therapy in melanoma. Cancer Cell. 2020;38(4):500–515.e3. doi: 10.1016/j.ccell.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faure M., Villiers C.L., Marche P.N. Normal differentiation and functions of mouse dendritic cells derived from RAG-deficient bone marrow progenitors. Cell Immunol. 2004;228(1):8–14. doi: 10.1016/j.cellimm.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Oberkampf M., Guillerey C., Mouriès J., et al. Mitochondrial reactive oxygen species regulate the induction of CD8+ T cells by plasmacytoid dendritic cells. Nat Commun. 2018;9(1):2241. doi: 10.1038/s41467-018-04686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanson M.C., Crespo M.P., Abraham W., et al. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J Clin Invest. 2015;125(6):2532–2546. doi: 10.1172/JCI79915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irvine D.J., Aung A., Silva M. Controlling timing and location in vaccines. Adv Drug Deliv Rev. 2020;158:91–115. doi: 10.1016/j.addr.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melamed J.R., Yerneni S.S., Arral M.L., et al. Ionizable lipid nanoparticles deliver mRNA to pancreatic β cells via macrophage-mediated gene transfer. Sci Adv. 2023;9(4) doi: 10.1126/sciadv.ade1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fitzgerald K.A., Kagan J.C. Toll-like receptors and the control of immunity. Cell. 2020;180(6):1044–1066. doi: 10.1016/j.cell.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiaie S.H., Majidi Zolbanin N., Ahmadi A., et al. Recent advances in mRNA-LNP therapeutics: immunological and pharmacological aspects. J Nanobiotechnology. 2022;20(1):276. doi: 10.1186/s12951-022-01478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cervantes J.L., Weinerman B., Basole C., Salazar J.C. TLR8: the forgotten relative revindicated. Cell Mol Immunol. 2012;9(6):434–438. doi: 10.1038/cmi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romero M.M., Basile J.I., Corra Feo L., López B., Ritacco V., Alemán M. Reactive oxygen species production by human dendritic cells involves TLR2 and dectin-1 and is essential for efficient immune response against Mycobacteria. Cell Microbiol. 2016;18(6):875–886. doi: 10.1111/cmi.12562. [DOI] [PubMed] [Google Scholar]
- 52.Dean I., Lee C.Y.C., Tuong Z.K., et al. Rapid functional impairment of natural killer cells following tumor entry limits anti-tumor immunity. Nat Commun. 2024;15(1):683. doi: 10.1038/s41467-024-44789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schiavoni G., Mattei F., Gabriele L. Type I interferons as stimulators of DC-mediated cross-priming: impact on anti-tumor response. Front Immunol. 2013;4:483. doi: 10.3389/fimmu.2013.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Sluis T.C., Sluijter M., van Duikeren S., et al. Therapeutic peptide vaccine-induced CD8 T cells strongly modulate intratumoral macrophages required for tumor regression. Cancer Immunol Res. 2015;3(9):1042–1051. doi: 10.1158/2326-6066.CIR-15-0052. [DOI] [PubMed] [Google Scholar]
- 55.Murray P.J. Immune regulation by monocytes. Semin Immunol. 2018;35:12–18. doi: 10.1016/j.smim.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Guo M., Liu M.Y.R., Brooks D.G. Regulation and impact of tumor-specific CD4+ T cells in cancer and immunotherapy. Trends Immunol. 2024;45(4):303–313. doi: 10.1016/j.it.2024.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Quezada S.A., Simpson T.R., Peggs K.S., et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitano S., Tsuji T., Liu C., et al. Enhancement of tumor-reactive cytotoxic CD4+ T cell responses after ipilimumab treatment in four advanced melanoma patients. Cancer Immunol Res. 2013;1(4):235–244. doi: 10.1158/2326-6066.CIR-13-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oh D.Y., Kwek S.S., Raju S.S., et al. Intratumoral CD4+ T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell. 2020;181(7):1612–1625.e13. doi: 10.1016/j.cell.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber J.S., Carlino M.S., Khattak A., et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): a randomised, phase 2b study. Lancet. 2024;403(10427):632–644. doi: 10.1016/S0140-6736(23)02268-7. [DOI] [PubMed] [Google Scholar]
- 61.Lorentzen C.L., Haanen J.B., Met Ö., Svane I.M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022;23(10):e450–e458. doi: 10.1016/S1470-2045(22)00372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garzia I., Nocchi L., Avalle L., et al. Tumor burden dictates the neoantigen features required to generate an effective cancer vaccine. Cancer Immunol Res. 2024;12(4):440–452. doi: 10.1158/2326-6066.CIR-23-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu Z., Leet D.E., Allesøe R.L., et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat Med. 2021;27(3):515–525. doi: 10.1038/s41591-020-01206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gang M., Wong P., Berrien-Elliott M.M., Fehniger T.A. Memory-like natural killer cells for cancer immunotherapy. Semin Hematol. 2020;57(4):185–193. doi: 10.1053/j.seminhematol.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ni J., Miller M., Stojanovic A., Garbi N., Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209(13):2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun J.C., Beilke J.N., Lanier L.L. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khalifa A.M., Nakamura T., Sato Y., Harashima H. Vaccination with a combination of STING agonist-loaded lipid nanoparticles and CpG-ODNs protects against lung metastasis via the induction of CD11bhighCD27low memory-like NK cells. Exp Hematol Oncol. 2024;13(1):36. doi: 10.1186/s40164-024-00502-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.