Abstract
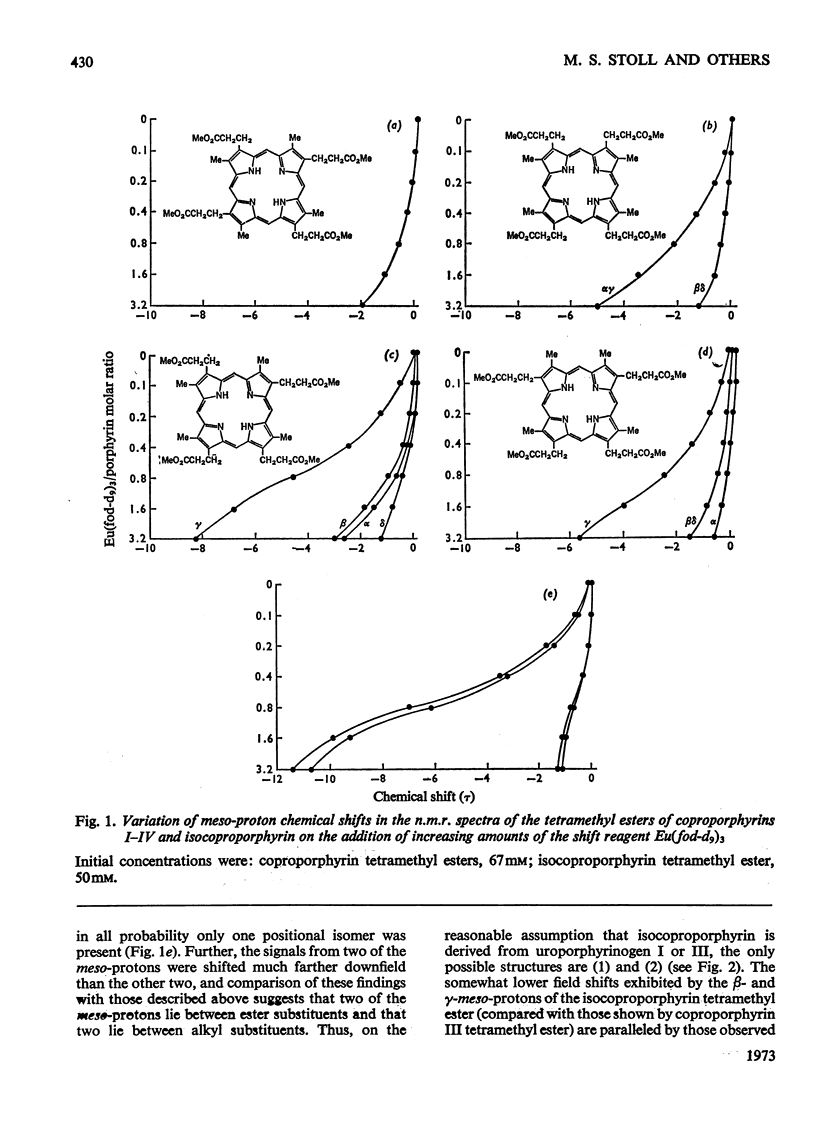
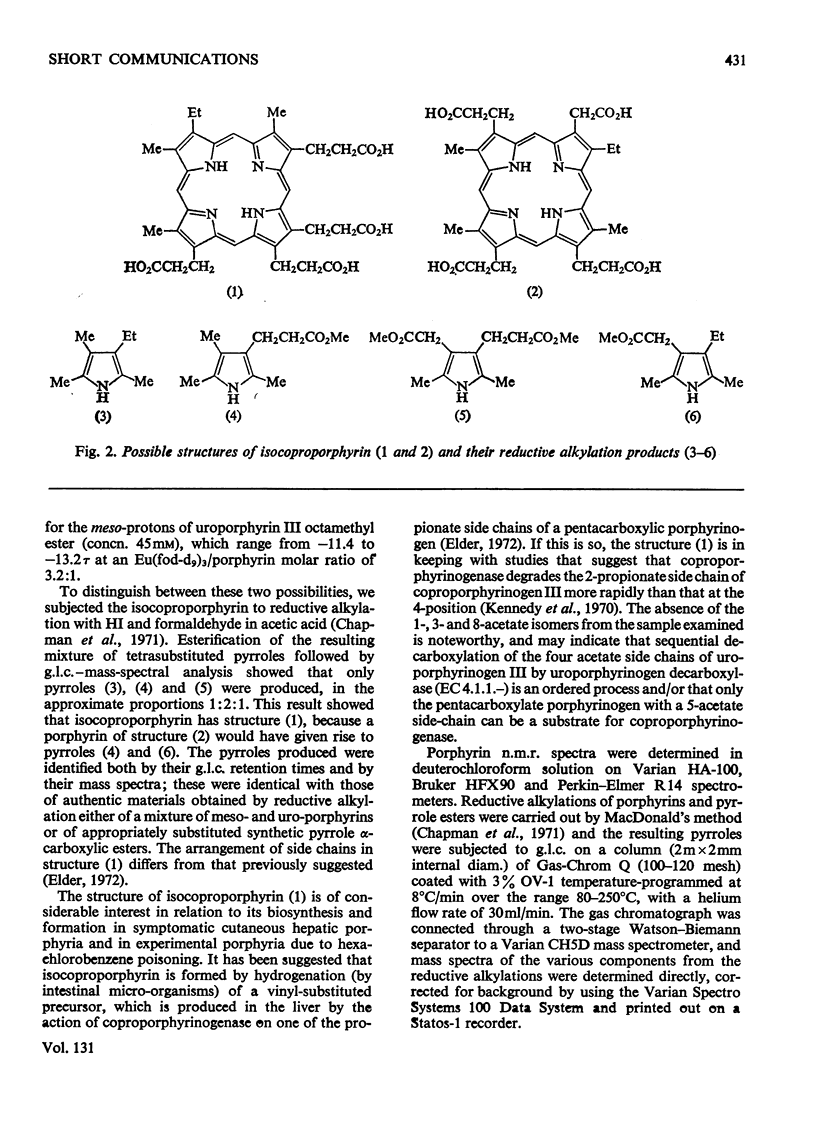
The use of `shift reagents' in the determination of n.m.r. spectra, and of reductive alkylation in combination with g.l.c.–mass spectrometry, facilitates assignment of the order of substituents in porphyrins, and the application of these new techniques to isocoproporphyrin is described.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elder G. H. Identification of a group of tetracarboxylate porphyrins, containing one acetate and three propionate -substituents, in faeces from patients with symptomatic cutaneous hepatic porphyria and from rats with porphyria due to hexachlorobenzene. Biochem J. 1972 Feb;126(4):877–891. doi: 10.1042/bj1260877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. H., Kenner G. W., Wass J. Pyrroles and related compounds. XX. Syntheses of coproporphyrins. J Chem Soc Perkin 1. 1972;12:1475–1483. doi: 10.1039/p19720001475. [DOI] [PubMed] [Google Scholar]
- Kennedy G. Y., Jackson A. H., Kenner G. W., Suckling C. J. Isolation, structure and synthesis of a tricarboxylic porphyrin from the harderian glands of the rat. FEBS Lett. 1970 Jan 15;6(1):9–12. doi: 10.1016/0014-5793(70)80027-8. [DOI] [PubMed] [Google Scholar]


