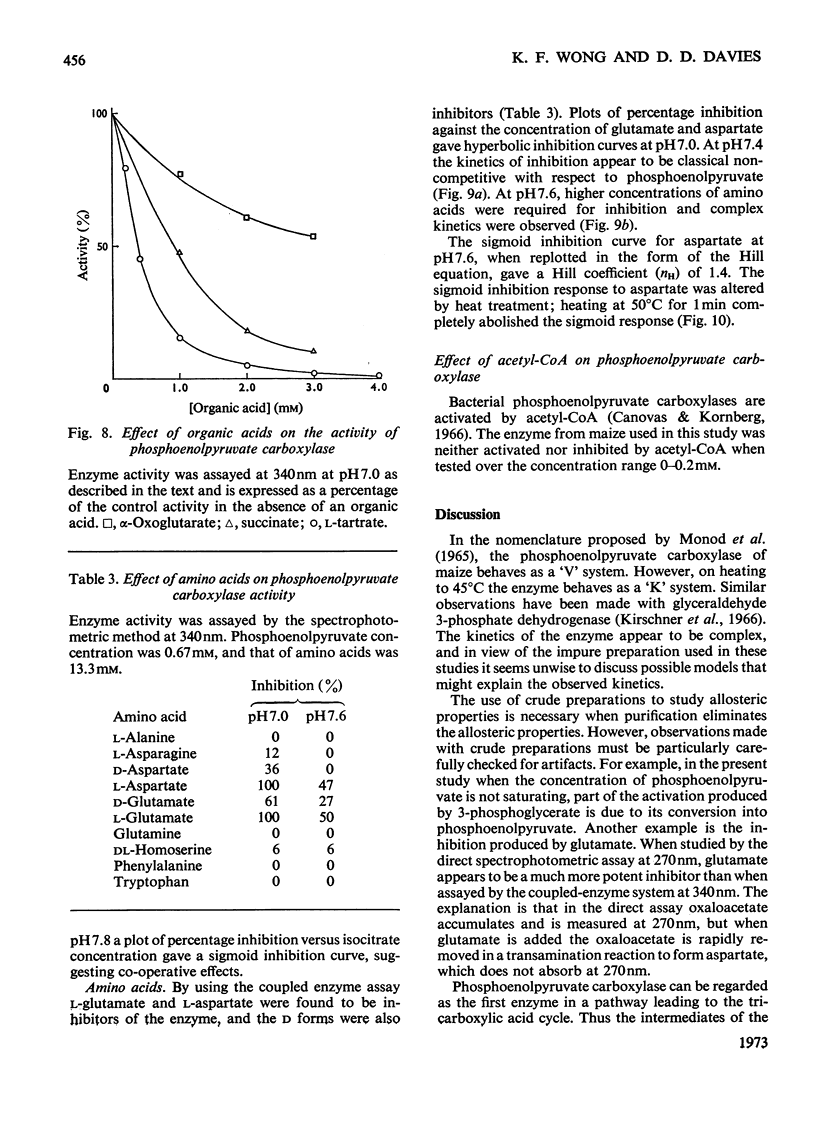
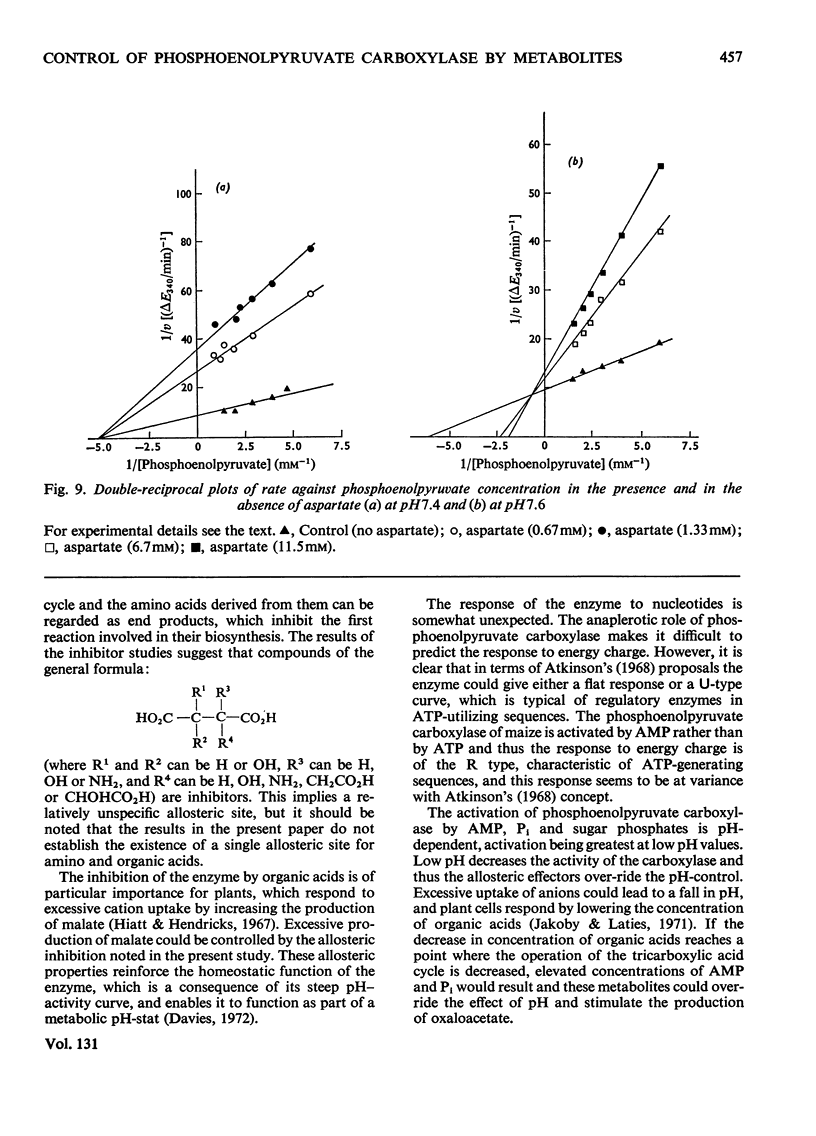
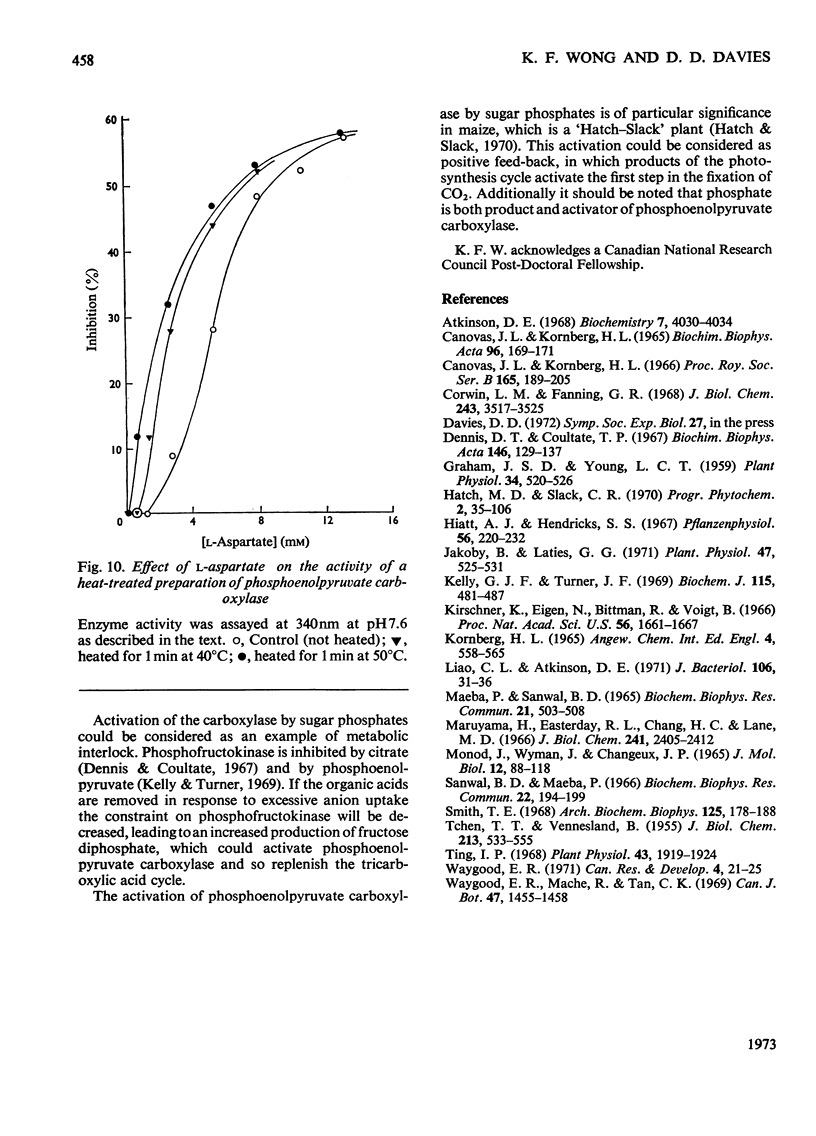
Abstract
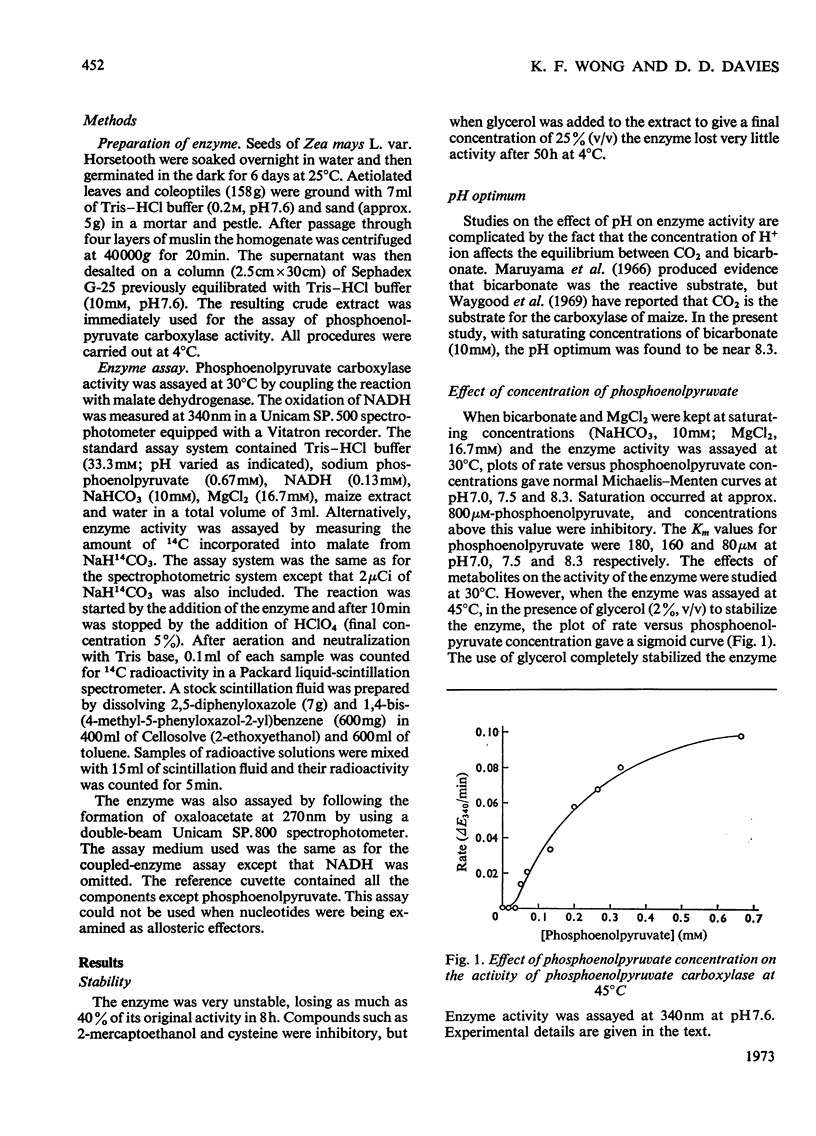
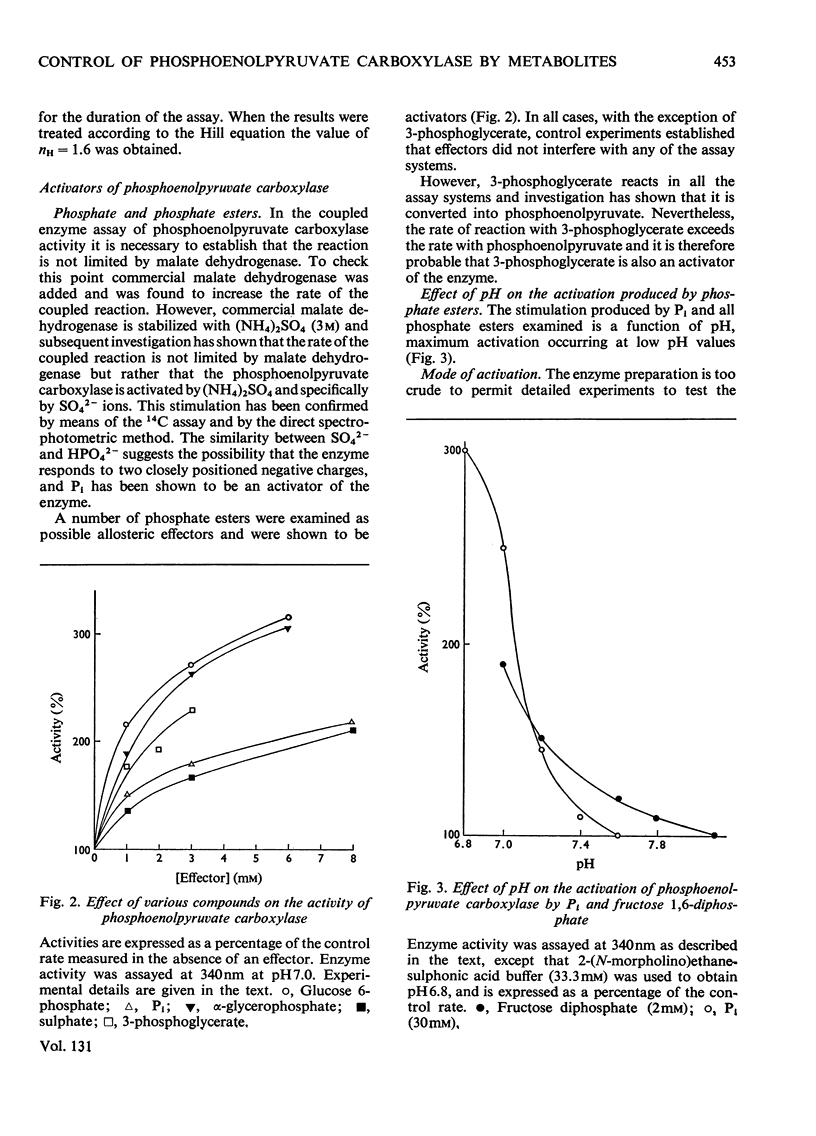
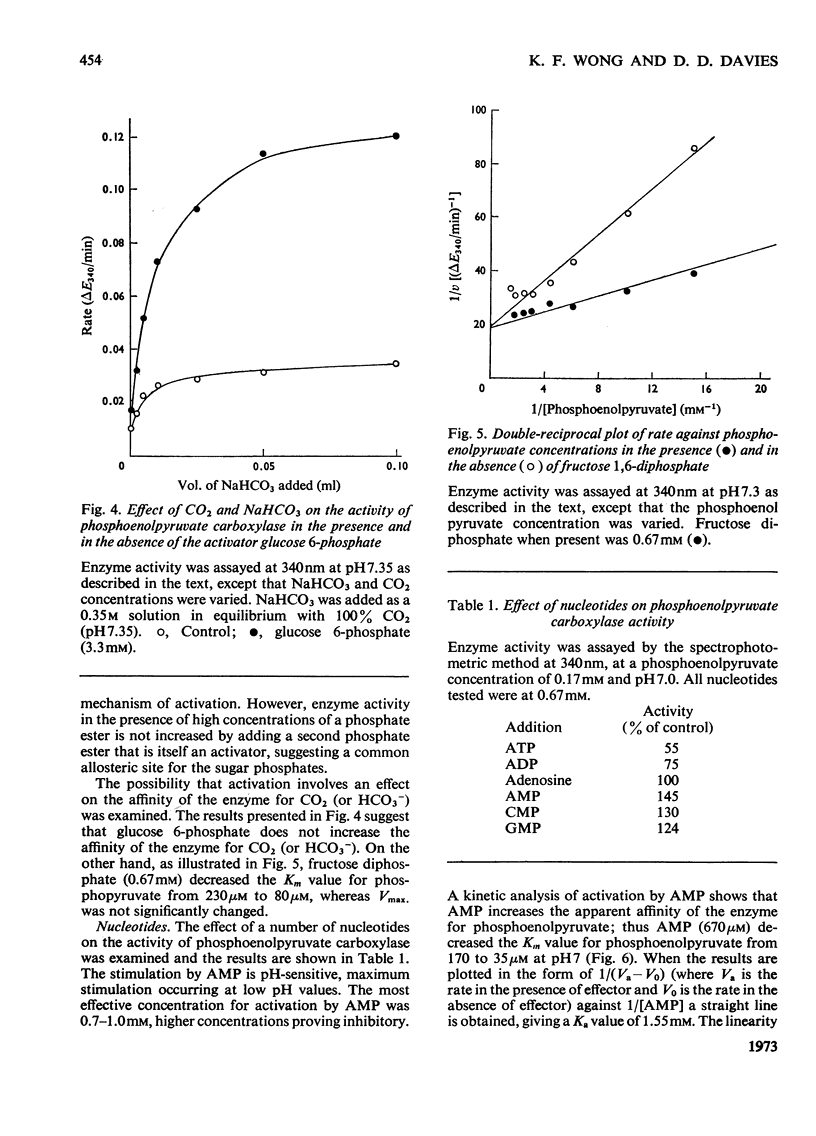
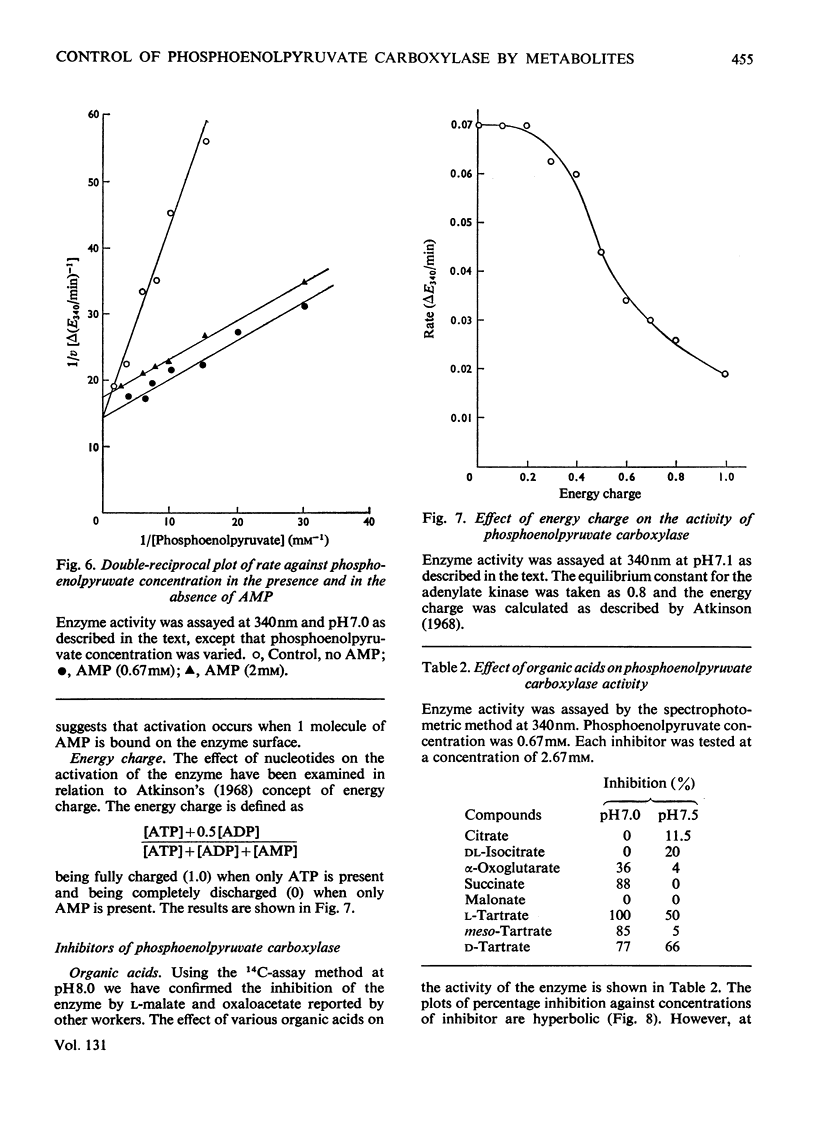
Crude preparations of phosphoenolpyruvate carboxylase obtained from aetiolated seedlings of Zea mays are unstable but can be stabilized with glycerol. At the pH optimum of 8.3, the Km value for phosphoenolpyruvate is 80μm. When assayed at 30°C, the enzyme shows normal Michaelis–Menten kinetics, but when assayed at 45°C sigmoid kinetics are exhibited. At pH7.0 the enzyme is inhibited by a number of dicarboxylic acids and by glutamate and aspartate. d and l forms of the hydroxy acids and amino acids are inhibitory and the kinetics approximate to simple non-competitive inhibition. The same compounds produce less inhibition at pH7.6 than at pH7.0 and the kinetics of inhibition are more complex. The enzyme is activated by Pi, by SO42− and by a number of sugar phosphates. Maximum activation occurs at acid pH values, where enzyme activity is lowest. The enzyme is activated by AMP and inhibited by ADP and ATP so that the response to energy charge is of the R type and is thus at variance with Atkinson's (1968) concept of energy charge. The physiological significance of the response to metabolites is discussed.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- CANOVAS J. L., KORNBERG H. L. FINE CONTROL OF PHOSPHOPYRUVATE CARBOXYLASE ACTIVITY IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Jan;96:169–172. doi: 10.1016/0005-2787(65)90624-6. [DOI] [PubMed] [Google Scholar]
- Corwin L. M., Fanning G. R. Studies of parameters affecting the allosteric nature of phosphoenolpyruvate carboxylase of Escherichia coli. J Biol Chem. 1968 Jun 25;243(12):3517–3525. [PubMed] [Google Scholar]
- Cánovas J. L., Kornberg H. L. Properties and regulation of phosphopyruvate carboxylase activity in Escherichia coli. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):189–205. doi: 10.1098/rspb.1966.0064. [DOI] [PubMed] [Google Scholar]
- Dennis D. T., Coultate T. P. The regulatory properties of a plant phosphofructokinase during leaf development. Biochim Biophys Acta. 1967 Sep 12;146(1):129–137. doi: 10.1016/0005-2744(67)90079-4. [DOI] [PubMed] [Google Scholar]
- Graham J. S., Young L. C. Fixation of Carbon Dioxide in Particulate Preparations from Barley Roots. Plant Physiol. 1959 Sep;34(5):520–526. doi: 10.1104/pp.34.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby B., Laties G. G. Bicarbonate Fixation and Malate Compartmentation in Relation to Salt-induced Stoichiometric Synthesis of Organic Acid. Plant Physiol. 1971 Apr;47(4):525–531. doi: 10.1104/pp.47.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Turner J. F. The regulation of pea-seed phosphofructokinase by phosphoenolpyruvate. Biochem J. 1969 Nov;115(3):481–487. doi: 10.1042/bj1150481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner K., Eigen M., Bittman R., Voigt B. The binding of nicotinamide-adenine dinucleotide to yeast d-glyceraldehyde-3-phosphate dehydrogenase: temperature-jump relaxation studies on the mechanism of an allosteric enzyme. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1661–1667. doi: 10.1073/pnas.56.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C. L., Atkinson D. E. Regulation at the phosphoenolpyruvate branchpoint in Azotobacter vinelandii: phosphoenolpyruvate carboxylase. J Bacteriol. 1971 Apr;106(1):31–36. doi: 10.1128/jb.106.1.31-36.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Maeba P., Sanwal B. D. Feedback inhibition of phosphoenolpyruvate carboxylase of Salmonella. Biochem Biophys Res Commun. 1965 Dec 9;21(5):503–508. doi: 10.1016/0006-291x(65)90412-2. [DOI] [PubMed] [Google Scholar]
- Maruyama H., Easterday R. L., Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. I. Purification and properties of phosphoenolpyruvate carboxylase. J Biol Chem. 1966 May 25;241(10):2405–2412. [PubMed] [Google Scholar]
- Sanwal B. D., Maeba P. Regulation of the activity of phosphoenolypyruvate carboxylase by fructose diphosphate. Biochem Biophys Res Commun. 1966 Jan 24;22(2):194–199. doi: 10.1016/0006-291x(66)90431-1. [DOI] [PubMed] [Google Scholar]
- Smith T. E. Partial purification and characteristics of potato phosphoenolpyruvate carboxylase. Arch Biochem Biophys. 1968 Apr;125(1):178–188. doi: 10.1016/0003-9861(68)90653-x. [DOI] [PubMed] [Google Scholar]
- TCHEN T. T., LOEWUS F. A., VENNESLAND B. The mechanism of enzymatic carbon dioxide fixation into oxal-acetate. J Biol Chem. 1955 Apr;213(2):547–555. [PubMed] [Google Scholar]
- Ting I. P. CO(2) Metabolism in Corn Roots. III. Inhibition of P-enolpyruvate Carboxylase by l-malate. Plant Physiol. 1968 Dec;43(12):1919–1924. doi: 10.1104/pp.43.12.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]


