Abstract
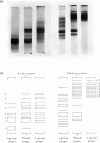
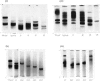
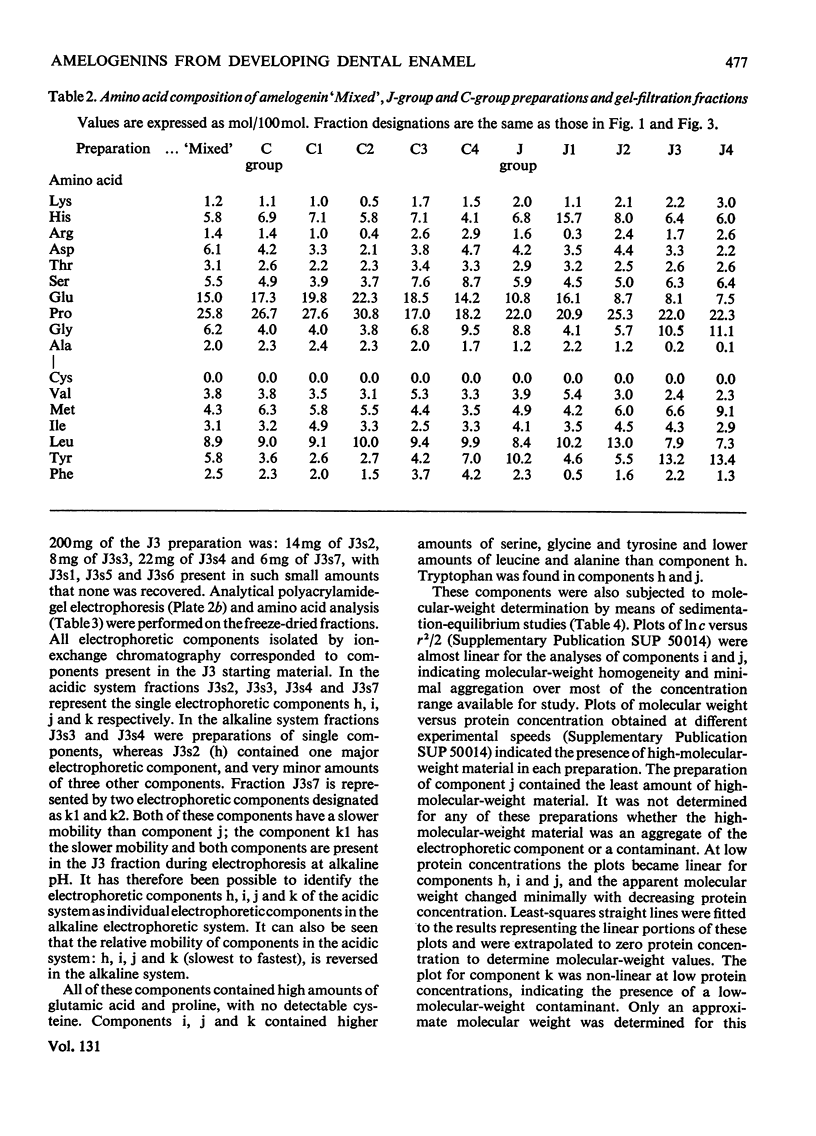
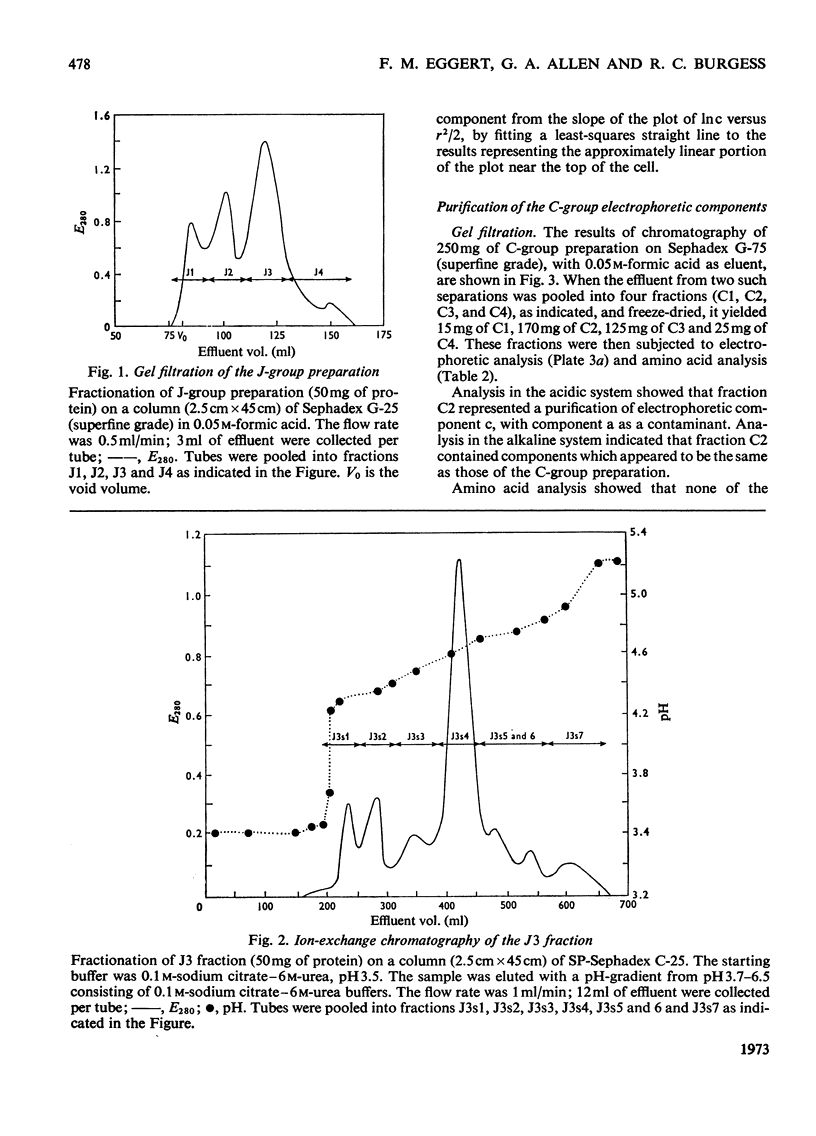
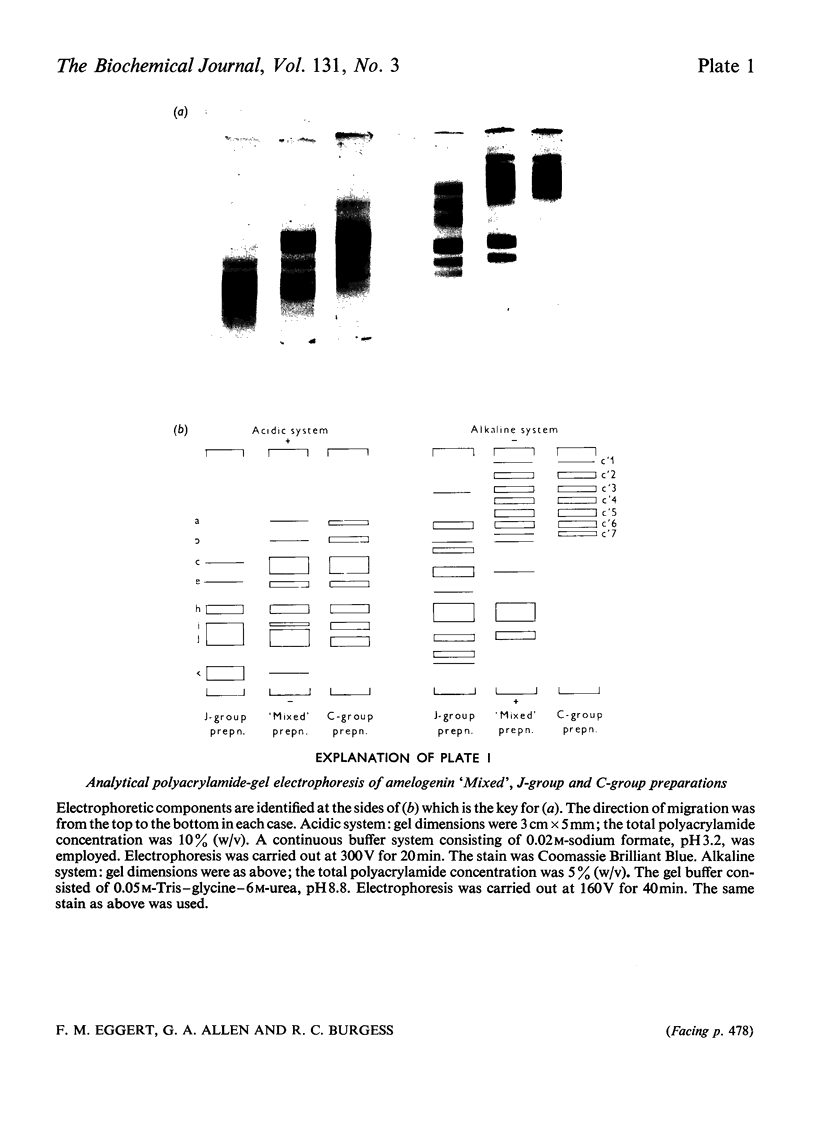
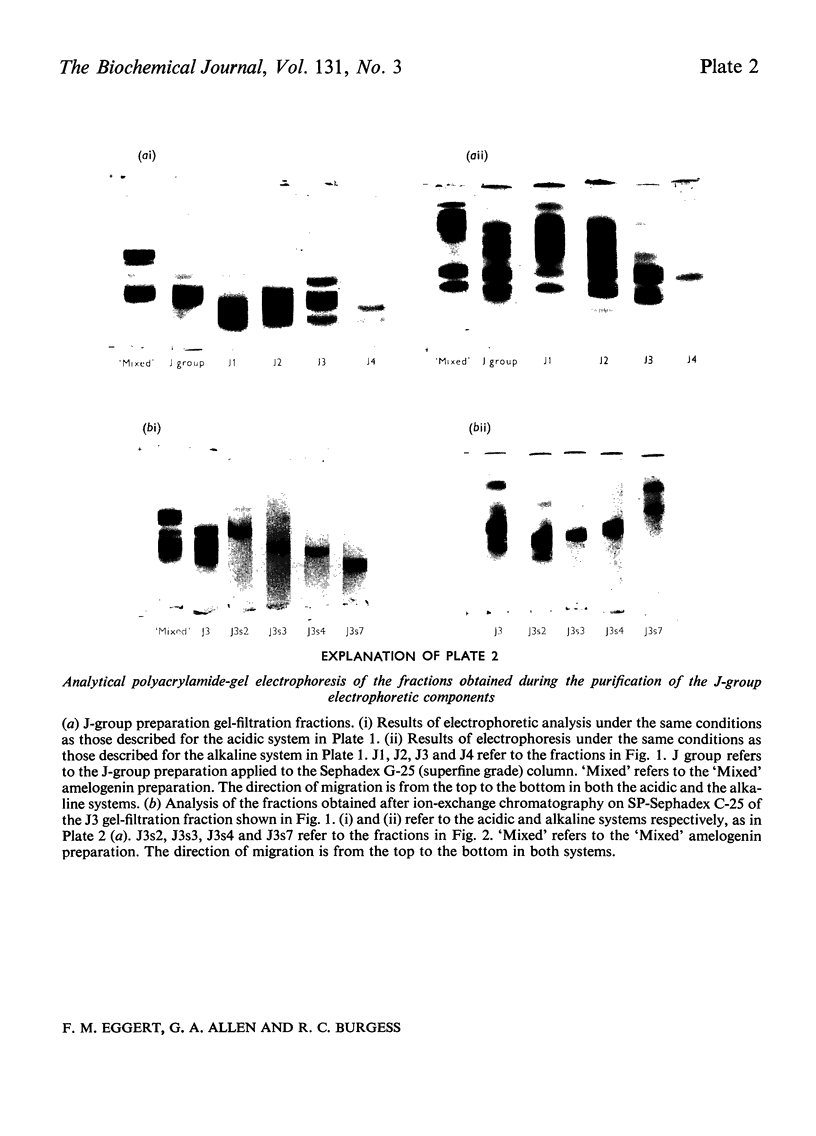
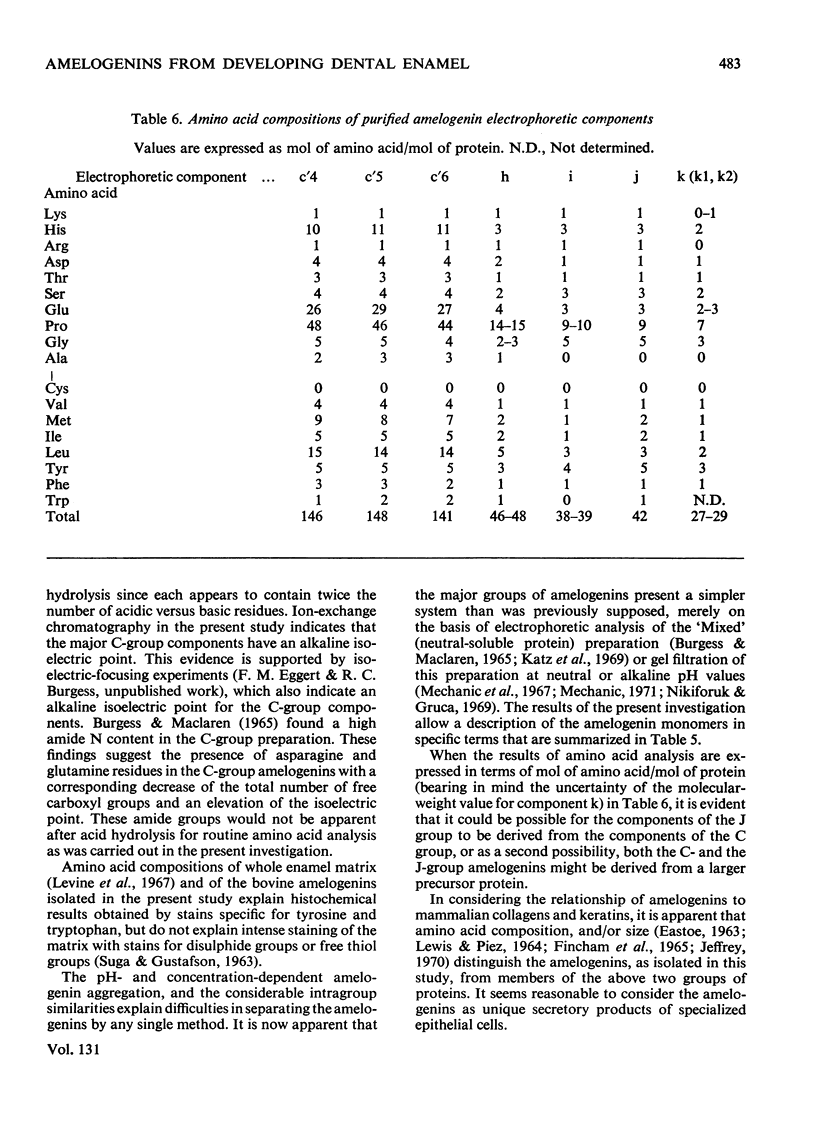
1. Procedures are described for the purification of amelogenin electrophoretic components and their analysis for homogeneity by polyacrylamide-gel electrophoresis at both acidic and alkaline pH values. 2. Most of these components belonged to two main groups, termed the J group and the C group after their major electrophoretic components. Sodium dodecyl sulphate-polyacrylamide-gel electrophoresis indicated that, within each group, proteins were of similar size, but the C-group proteins were larger than those of the J group. 3. By sedimentation-equilibrium ultracentrifugation and amino acid analysis, the four J-group components were found to be very small proteins (mol. wt. 5500–3000) and, except for one, similar in amino acid composition. The components of the C group were found to be proteins of moderate size (mol. wt. 16800–16100) with very similar amino acid compositions. A third minor amelogenin group of intermediate size was also found, but not further analysed. Details of the results of the ultracentrifuge studies are given in a supplementary paper that has been deposited as Supplementary Publication SUP 50014 at the National Lending Library for Science and Technology, Boston Spa, Yorks. LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1973) 131, 5. 4. Two of the J-group components were similar to amelogenins isolated by other workers. 5. All amelogenins analysed were rich in proline, glutamic acid, histidine and methionine, and contained no half-cystine. Their amino acid compositions, combined with their molecular weights, serve to distinguish the amelogenins from both collagens and keratins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- EASTOE J. E. Organic matrix of tooth enamel. Nature. 1960 Jul 30;187:411–412. doi: 10.1038/187411b0. [DOI] [PubMed] [Google Scholar]
- EASTOE J. E. THE AMINO ACID COMPOSITION OF PROTEINS FROM THE ORAL TISSUES. II. THE MATRIX PROTEINS IN DENTINE AND ENAMEL FROM DEVELOPING HUMAN DECIDUOUS TEETH. Arch Oral Biol. 1963 Sep-Oct;8:633–652. doi: 10.1016/0003-9969(63)90078-5. [DOI] [PubMed] [Google Scholar]
- EASTOE J. E. THE CHEMICAL COMPOSITION OF BONE AND TOOTH. Adv Fluorine Res. 1965;21:5–17. [PubMed] [Google Scholar]
- Fincham A. G. Electrophoretic and Sephadex gel filtration studies of bovine foetal enamel matrix at acid pH. Calcif Tissue Res. 1968 Dec 18;2(4):353–360. doi: 10.1007/BF02279223. [DOI] [PubMed] [Google Scholar]
- GLIMCHER M. J., MECHANIC G., BONAR L. C., DANIEL E. J. The amino acid composition of the organic matrix of decalcified fetal bovine dental enamel. J Biol Chem. 1961 Dec;236:3210–3213. [PubMed] [Google Scholar]
- Glimcher M. J., Mechanic G. L., Friberg U. A. The amino acid composition of the organic matrix and the neutral-soluble and acid-soluble components of embryonic bovine enamel. Biochem J. 1964 Oct;93(1):198–202. doi: 10.1042/bj0930198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey P. D. The molecular weights of two reduced and carboxymethylated keratins by disk gel electrophoresis and a comparison of two methods of analysing the results. Aust J Biol Sci. 1970 Aug;23(4):809–819. doi: 10.1071/bi9700809. [DOI] [PubMed] [Google Scholar]
- KUPKE D. W. Osmometry on insulin and ribonuclease in dissociating media. C R Trav Lab Carlsberg. 1961;32:107–124. [PubMed] [Google Scholar]
- Kaneko I. Proteins in embryonic bovine enamel. Bull Tokyo Med Dent Univ. 1967 Mar;14(1):105–112. [PubMed] [Google Scholar]
- Katz E. P., Seyer J., Levine P. T., Glimcher M. J. The comparative biochemistry of the organic matrix of developing enamel--II. Ultracentrifugal and electrophoretic characterization of proteins soluble at neutral pH. Arch Oral Biol. 1969 May;14(5):533–539. doi: 10.1016/0003-9969(69)90146-0. [DOI] [PubMed] [Google Scholar]
- LEWIS M. S., PIEZ K. A. SEDIMENTATION-EQUILIBRIUM STUDIES OF THE MOLECULAR WEIGHT OF SINGLE AND DOUBLE CHAINS FROM RAT-SKIN COLLAGEN. Biochemistry. 1964 Aug;3:1126–1131. doi: 10.1021/bi00896a020. [DOI] [PubMed] [Google Scholar]
- Levine P. T., Seyer J., Huddleston J., Glimcher M. J. The comparative biochemistry of the organic matrix proteins of developing enamel. I. Amino acid composition. Arch Oral Biol. 1967 Mar;12(3):407–410. doi: 10.1016/0003-9969(67)90225-7. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., KIMMEL J. R., HILL R. L., SCHMIDT W. R. Amino acid composition of horse heart cytochrome c. J Biol Chem. 1962 Jul;237:2148–2150. [PubMed] [Google Scholar]
- Maroux S., Rovery M. Contribution à l'etude de la structure de la chymotrypsine alpha de boeuf. La chaîne C et son core trypsique. Biochim Biophys Acta. 1966 Jan 11;113(1):126–143. [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- Mechanic G. L., Katz E. P., Glimcher M. J. The Sephadex gel filtration characteristics of the neutral soluble proteins of embryonic bovine enamel. Biochim Biophys Acta. 1967 Jan 18;133(1):97–113. doi: 10.1016/0005-2795(67)90042-6. [DOI] [PubMed] [Google Scholar]
- Nikiforuk G., Gruca M. Immunological reactions of the organic matrix of developing bovine enamel. Calcif Tissue Res. 1969;4(2):129–135. doi: 10.1007/BF02279114. [DOI] [PubMed] [Google Scholar]
- Nikiforuk G., Simmons N. S. Purification and properties of protein from embryonic bovine enamel. J Dent Res. 1965 Nov-Dec;44(6 Suppl):1119–1122. doi: 10.1177/00220345650440061101. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A. Amino acid composition of some calcified proteins. Science. 1961 Sep 22;134(3482):841–842. doi: 10.1126/science.134.3482.841. [DOI] [PubMed] [Google Scholar]
- Schachman H. K., Edelstein S. J. Ultracentrifuge studies with absorption optics. IV. Molecular weight determinations at the microgram level. Biochemistry. 1966 Aug;5(8):2681–2705. doi: 10.1021/bi00872a029. [DOI] [PubMed] [Google Scholar]
- Seyer J. M., Glimcher M. J. The isolation of phosphorylated polypeptide components of the organic matrix of embryonic bovine enamel. Biochim Biophys Acta. 1971 Apr 27;236(1):279–291. doi: 10.1016/0005-2795(71)90177-2. [DOI] [PubMed] [Google Scholar]
- Seyer J., Glimcher M. J. The content and nature of the carbohydrate components of the organic matrix of embryonic bovine enamel. Biochim Biophys Acta. 1969 Sep 2;184(3):509–522. doi: 10.1016/0304-4165(69)90265-7. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]