Abstract
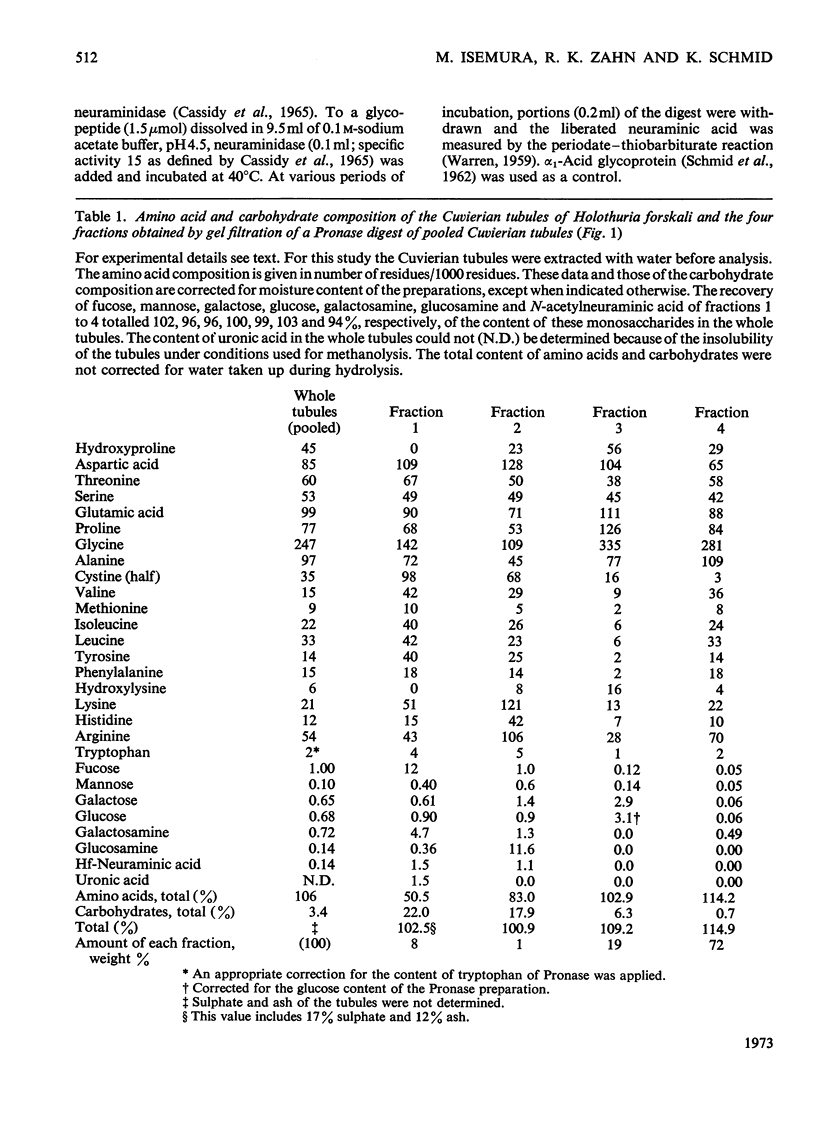
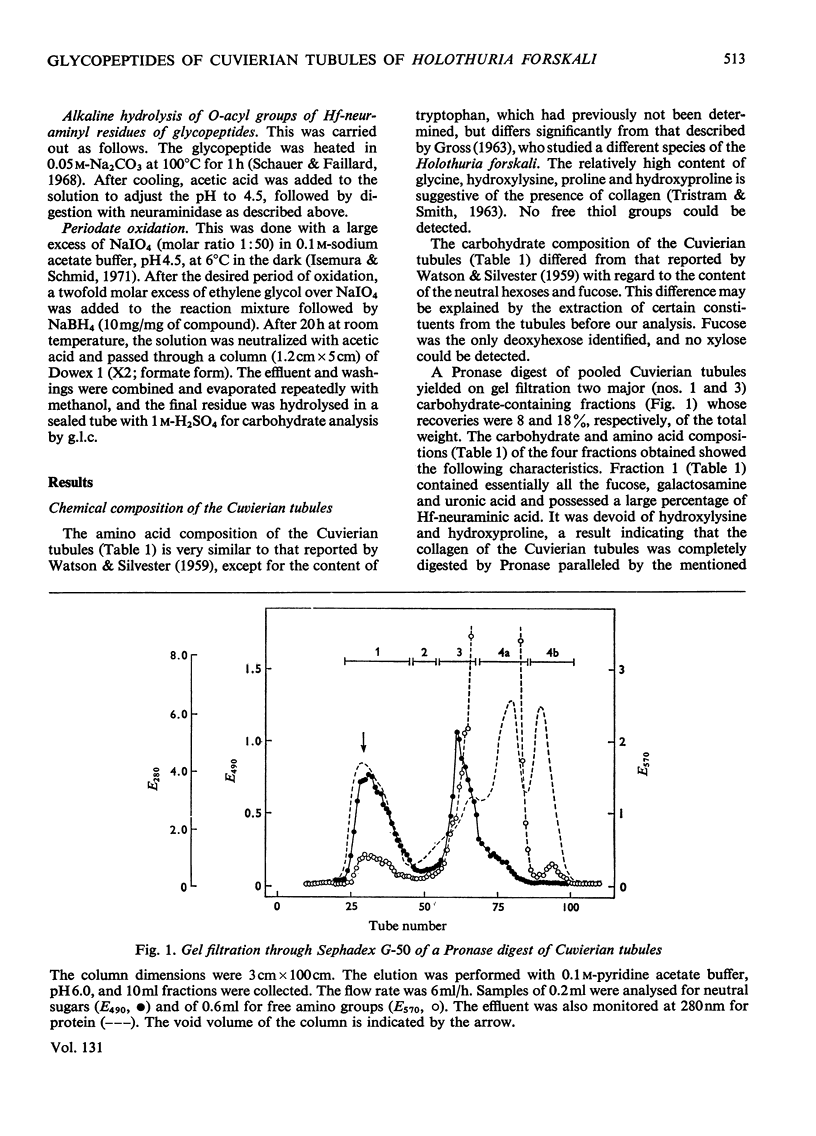
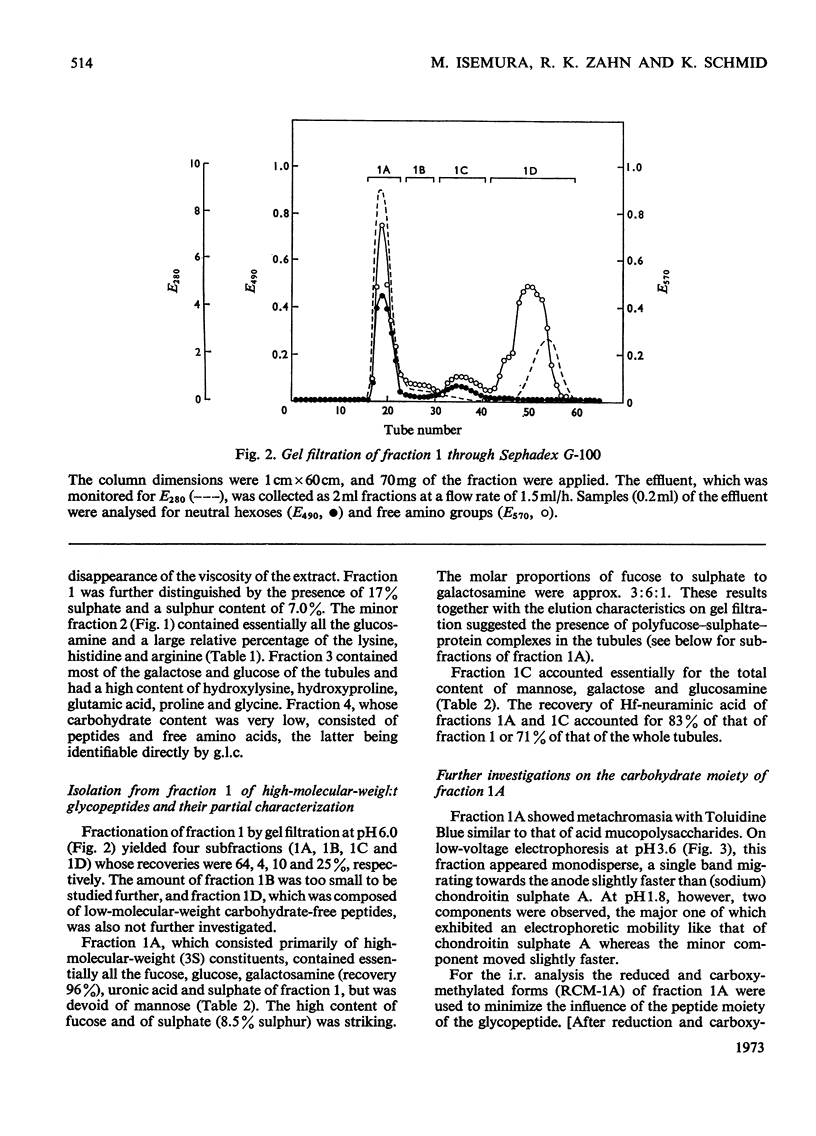
The Cuvierian tubules of Holothuria forskali Della Chiaje, a sea cucumber found in the Adriatic Sea, were investigated with regard to their carbohydrate moieties. From a Pronase digest of these tubules three types of carbohydrate units were isolated and characterized. 1. A high-molecular-weight glycopeptide fraction was shown to contain sulphated polyfucose, galactosamine, a uronic acid and a previously unknown neuraminic acid derivative. The sulphate was shown by i.r. analysis to be present as an O-ester. The carbohydrate unit was linked O-glycosidically to threonine and serine residues in the polypeptide chain. The hitherto unknown neuraminic acid derivative (Hf-neuraminic acid) was resistant to enzymic cleavage by neuraminidase, even after mild alkaline hydrolysis for the removal of O-acyl residues. However, the glycosidic linkage of this compound to the other part of the carbohydrate moiety was readily cleaved by mild acid hydrolysis. Its chromatographic properties distinguished Hf-neuraminic acid from other known neuraminic acid derivatives (N-acetyl-, NO-diacetyl-, NOO-triacetyl- and N-glycollyl-neuraminic acid). Further, this acidic sugar was shown to possess neuraminic acid as its basic structure. Thus, an as yet unknown substituent lends the distinct properties to Hf-neuraminic acid. 2. The carbohydrate composition of a second glycopeptide fraction consisting of a derivative of neuraminic acid, galactose, mannose and glucosamine was similar to that of the well-known carbohydrate groups of the globular glycoproteins. 3. The third fraction contained two glycopeptides containing the disaccharide, glucosylgalactose, which was shown to be linked to the hydroxyl group of hydroxylysine residues of a collagen-like protein. Approximately half of these residues were glycosylated. In addition to these glycopeptides, a small amount of a third glycopeptide that carried only a galactosyl residue was detected. The amino acid sequence of the two major compounds were found to be Gly-Ala-Hyl*-Gly-Ser and Gly-Pro-Hyl*-Gly-Asp, where Hyl* represents a glycosylated amino acid residue.
Full text
PDF



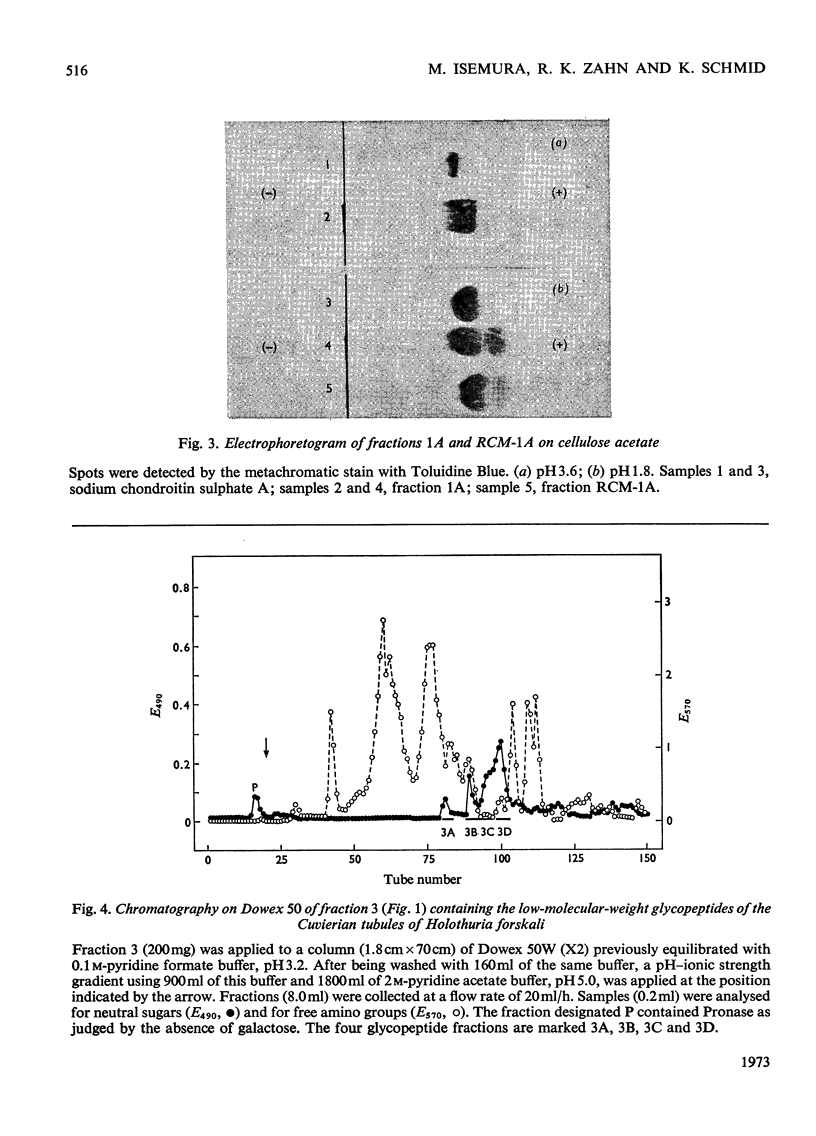
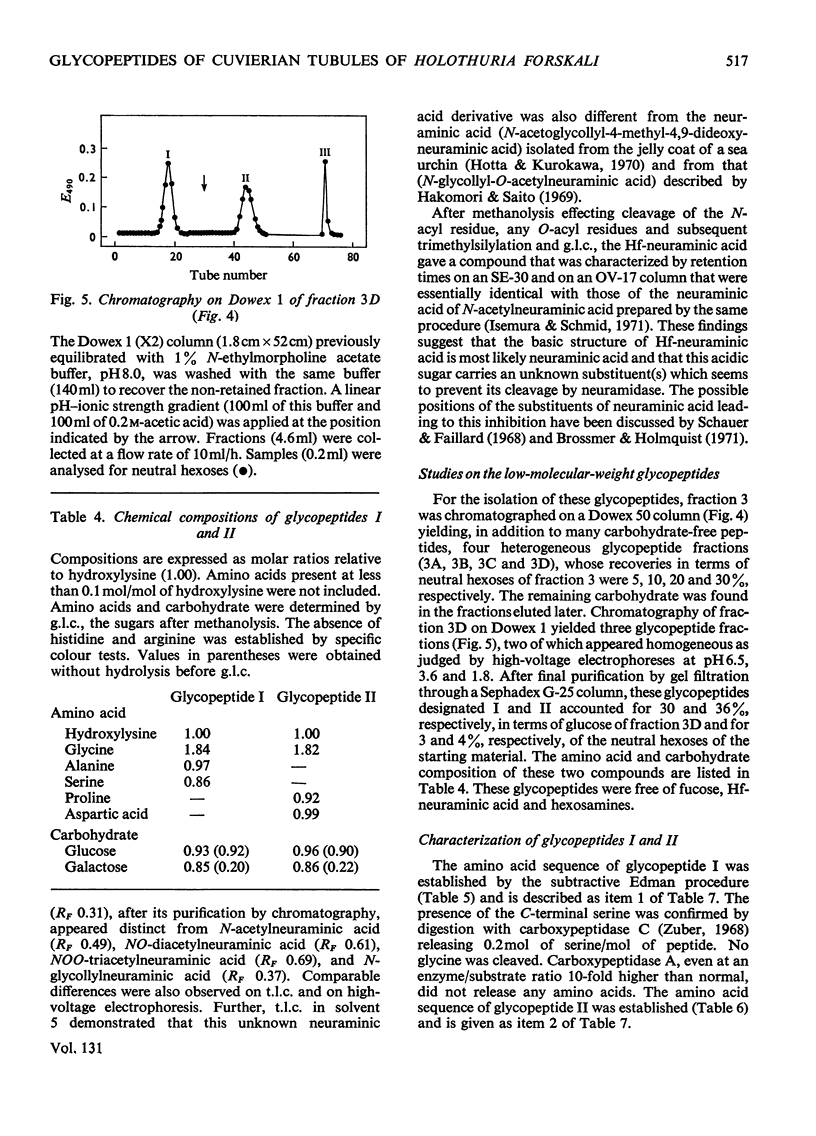
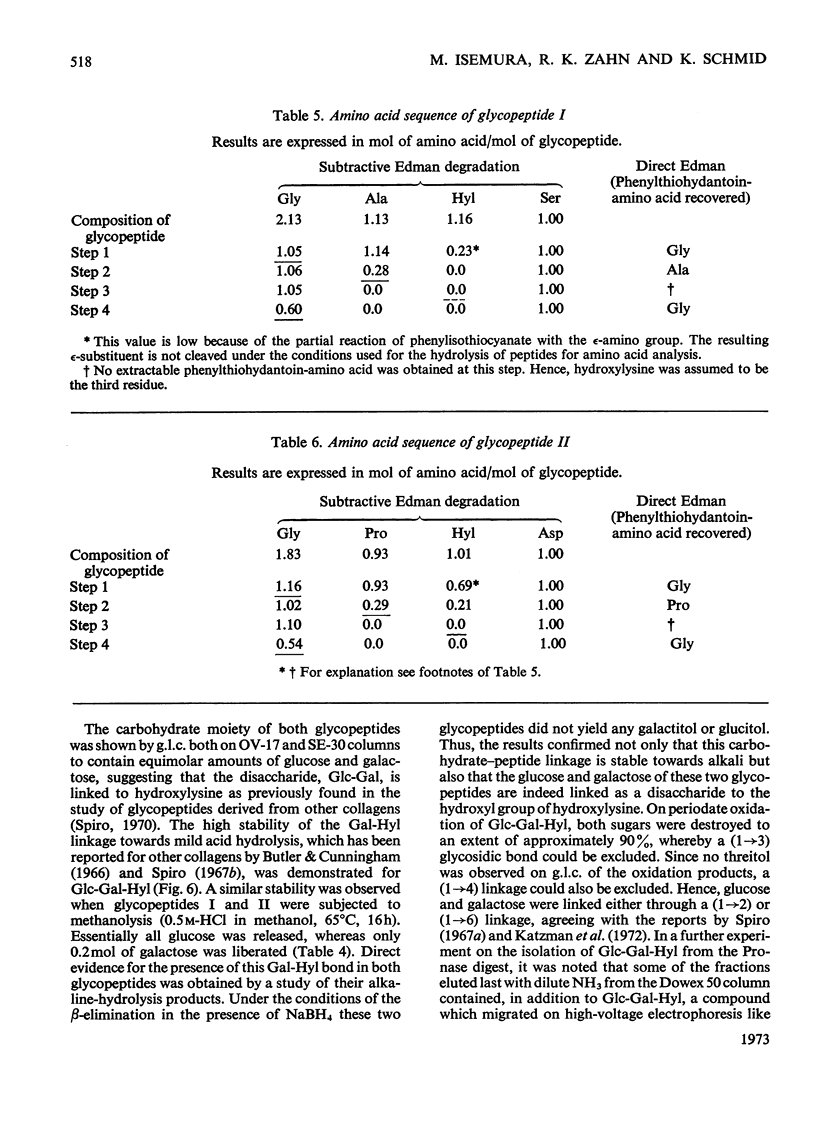
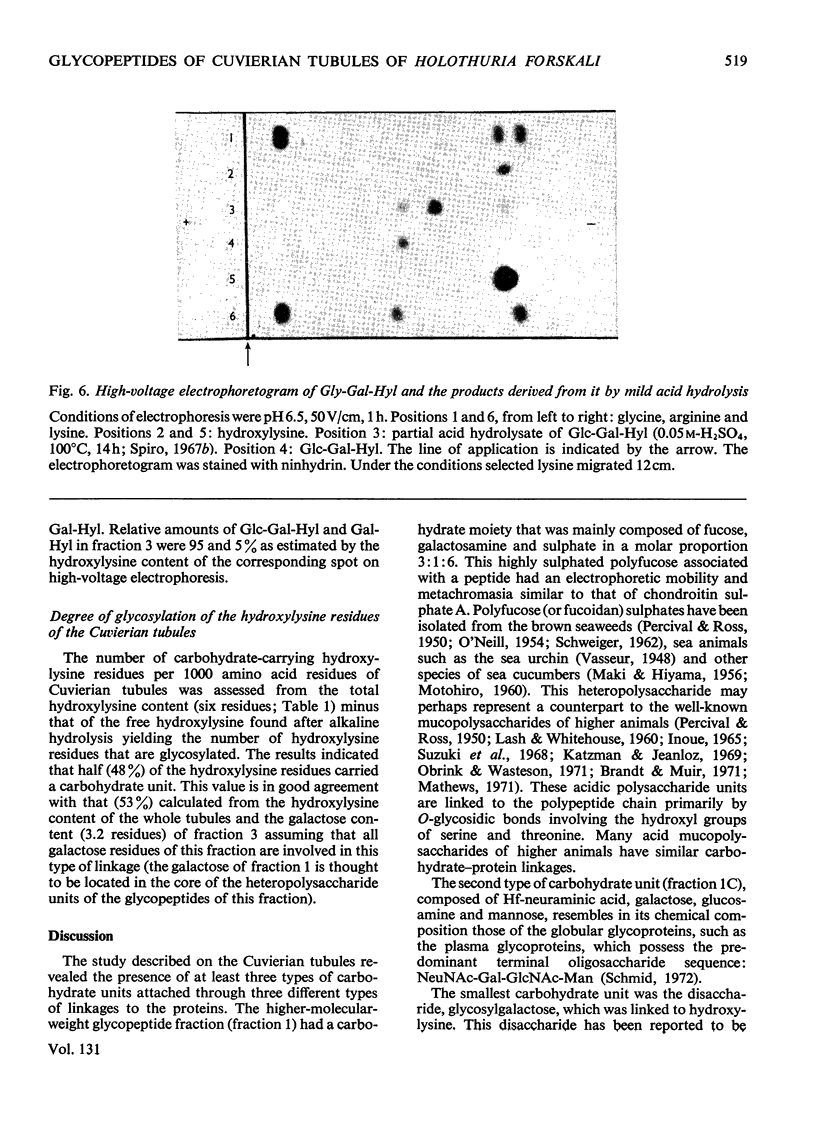


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J. A sensitive reagent for detecting 2-deoxysugars and 3-deoxypolyols. J Chromatogr. 1966 Jan;21(1):163–164. doi: 10.1016/s0021-9673(01)91287-7. [DOI] [PubMed] [Google Scholar]
- Brandt K. D., Muir H. Heterogeneity of protein-polysaccharides of porcine articular cartilage. The chondroitin sulphate proteins associaterd with collagen. Biochem J. 1971 Aug;123(5):747–755. doi: 10.1042/bj1230747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossmer R., Holmquist L. On the specificity of neuraminidase. Synthesis of 5-N-acetyl-D-nonulosamine (5-acetamido-3,5-dideoxy-D-glycera- -D-galacto-nonulose) and 5-N-acetyl-D-neuraminamide (5-acetamido-3,5-dideoxy-D-glycero- -D-galacto-nonulosonamide) and their methyl - and benzyl -ketosides. Hoppe Seylers Z Physiol Chem. 1971 Dec;352(12):1715–1719. doi: 10.1515/bchm2.1971.352.2.1715. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Cunningham L. W. Evidence for the linkage of a disaccharide to hydroxylysine in tropocollagen. J Biol Chem. 1966 Sep 10;241(17):3882–3888. [PubMed] [Google Scholar]
- Cassidy J. T., Jourdian G. W., Roseman S. The sialic acids. VI. Purification and properties of sialidase from Clostridium perfringens. J Biol Chem. 1965 Sep;240(9):3501–3506. [PubMed] [Google Scholar]
- Cunningham L. W., Ford J. D. A comparison of glycopeptides derived from soluble and insoluble collagens. J Biol Chem. 1968 May 10;243(9):2390–2398. [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B. Studies on sulphatases. V. The determination of inorganic sulphate in the study of sulphatases. Biochem J. 1953 Oct;55(3):436–440. doi: 10.1042/bj0550436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga M., Hirs C. H., Lai C. Y. Primary structure of bovine carboxypeptidase B. 3. The carboxyl-terminal sequence. Arch Biochem Biophys. 1968 Feb;123(2):353–360. doi: 10.1016/0003-9861(68)90145-8. [DOI] [PubMed] [Google Scholar]
- Hakomori S., Saito T. Isolation and characterization of a glycosphingolipid having a new sialic acid. Biochemistry. 1969 Dec;8(12):5082–5088. doi: 10.1021/bi00840a061. [DOI] [PubMed] [Google Scholar]
- Hotta K., Kurokawa M., Isaka S. Isolation and identification of two sialic acids from the jelly coat of sea urchin eggs. J Biol Chem. 1970 Dec 10;245(23):6307–6311. [PubMed] [Google Scholar]
- INOUE S. ISOLATION OF NEW POLYSACCHARIDE SULPHATES FROM CHARONIA LAMPAS. Biochim Biophys Acta. 1965 Mar 1;101:16–25. doi: 10.1016/0926-6534(65)90026-6. [DOI] [PubMed] [Google Scholar]
- Isemura M., Ikenaka T., Matsushima Y. Evidence for occurrence of a characteristic amino acid sequence of glycopeptides in the linkage region between peptide and carbohydrate. Biochem Biophys Res Commun. 1972 Jan 31;46(2):457–462. doi: 10.1016/s0006-291x(72)80160-8. [DOI] [PubMed] [Google Scholar]
- Iwanaga S., Wallén P., Gröndahl N. J., Henschen A., Blombäck B. On the primary structure of human fibrinogen. Isolation and characterization of N-terminal fragments from plasmic digests. Eur J Biochem. 1969 Mar;8(2):189–199. doi: 10.1111/j.1432-1033.1969.tb00514.x. [DOI] [PubMed] [Google Scholar]
- JEPSON J. B., SMITH I. Multiple dipping procedures in paper chromatography: a specific test for hydroxy-proline. Nature. 1953 Dec 12;172(4389):1100–1101. doi: 10.1038/1721100b0. [DOI] [PubMed] [Google Scholar]
- Katzman R. L., Halford M. H., Reinhold V. N., Jeanloz R. W. Isolation and structure determination of glucosylgalactosylhydroxylysine from sponge and sea anemone collagen. Biochemistry. 1972 Mar 28;11(7):1161–1167. doi: 10.1021/bi00757a008. [DOI] [PubMed] [Google Scholar]
- Katzman R. L., Jeanloz R. W. Acid polysaccharides from invertebrate connective tissue: phylogenetic aspects. Science. 1969 Nov 7;166(3906):758–759. doi: 10.1126/science.166.3906.758. [DOI] [PubMed] [Google Scholar]
- Kawai Y., Seno N., Anno K. Chondroitin polysulfate of squid cartilage. J Biochem. 1966 Sep;60(3):317–321. doi: 10.1093/oxfordjournals.jbchem.a128438. [DOI] [PubMed] [Google Scholar]
- LASH J. W., WHITEHOUSE M. W. An unusual polysaccharide in chondroid tissue of the snail Busycon: polyglucose sulphate. Biochem J. 1960 Feb;74:351–355. doi: 10.1042/bj0740351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANN T., LEONE E. Studies on the metabolism of semen. VIII. Ergothioneine as a normal constituent of boar seminal plasma; purification and crystallization; site of formation and function. Biochem J. 1953 Jan;53(1):140–148. doi: 10.1042/bj0530140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B. Comparative biochemistry of chondroitin sulphate-proteins of cartilage and notochord. Biochem J. 1971 Nov;125(1):37–46. doi: 10.1042/bj1250037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan P. H., Jacobs H. G., Segrest J. P., Cunningham L. W. A comparative study of glycopeptides derived from selected vertebrate collagens. A possible role of the carbohydrate in fibril formation. J Biol Chem. 1970 Oct 10;245(19):5042–5048. [PubMed] [Google Scholar]
- Obrink B., Wasteson A. Nature of the interaction of chondroitin 4-sulphate and chondroitin sulphate-proteoglycan with collagen. Biochem J. 1971 Jan;121(2):227–233. doi: 10.1042/bj1210227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnell S. R., Fox R., Krane S. M. Human collagens: differences in glycosylated hydroxylysines in skin and bone. Biochim Biophys Acta. 1971 Jan 19;229(1):119–122. doi: 10.1016/0005-2795(71)90325-4. [DOI] [PubMed] [Google Scholar]
- RIENITS K. G. The electrophoresis of acid mucopolysaccharides on filter paper. Biochem J. 1953 Jan;53(1):79–85. doi: 10.1042/bj0530079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer R., Faillard H. Zur Wirkungsspezifität der Neuraminidase. Das Verhalten isomerer N.O-Diacetylneuraminsäureglykoside im Submaxillarismucin von Pferd und Rind bei Einwirkung bakterieller Neuraminidase. Hoppe Seylers Z Physiol Chem. 1968 Aug;349(8):961–968. [PubMed] [Google Scholar]
- Schmid K., Okuyama T., Kaufmann H. Isolation and chemical composition of the human plasma alpha-1-acid glycoprotein variants. Biochim Biophys Acta. 1968 Apr 9;154(3):565–572. doi: 10.1016/0005-2795(68)90017-2. [DOI] [PubMed] [Google Scholar]
- Scholz R. W., Rhoades R. A. Lipid metabolism by rat lung in vitro. Effect of starvation and re-feeding on utilization of (U- 14 C)glucose by lung slices. Biochem J. 1971 Sep;124(2):257–264. doi: 10.1042/bj1240257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro R. G. Characterization and quantitative determination of the hydroxylysine-linked carbohydrate units of several collagens. J Biol Chem. 1969 Feb 25;244(4):602–612. [PubMed] [Google Scholar]
- Spiro R. G., Fukushi S. The lens capsule. Studies on the carbohydrate units. J Biol Chem. 1969 Apr 25;244(8):2049–2058. [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Annu Rev Biochem. 1970;39:599–638. doi: 10.1146/annurev.bi.39.070170.003123. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Studies on the renal glomerular basement membrane. Nature of the carbohydrate units and their attachment to the peptide portion. J Biol Chem. 1967 Apr 25;242(8):1923–1932. [PubMed] [Google Scholar]
- Spiro R. G. The structure of the disaccharide unit of the renal glomerular basement membrane. J Biol Chem. 1967 Oct 25;242(20):4813–4823. [PubMed] [Google Scholar]
- Suzuki S., Saito H., Yamagata T., Anno K., Seno N., Kawai Y., Furuhashi T. Formation of three types of disulfated disaccharides from chondroitin sulfates by chondroitinase digestion. J Biol Chem. 1968 Apr 10;243(7):1543–1550. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- TRISTRAM G. R., SMITH R. H. THE AMINO ACID COMPOSITION OF SOME PURIFIED PROTEINS. Adv Protein Chem. 1963;18:227–318. doi: 10.1016/s0065-3233(08)60270-3. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Bertolini M., Pigman W. Serine and threonine glycosidic linkages in bovine submaxillary mucin. Biochem Biophys Res Commun. 1964 Jul 27;16(5):404–409. doi: 10.1016/0006-291x(64)90366-3. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WATSON M. R. The chemical composition of earthworm cuticle. Biochem J. 1958 Mar;68(3):416–420. doi: 10.1042/bj0680416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walborg E. F., Jr, Christensson L., Gardell S. An ion-exchange column chromatographic method for the separation and quantitative analysis of neutral monosaccharides. Anal Biochem. 1965 Nov;13(2):177–185. doi: 10.1016/0003-2697(65)90187-9. [DOI] [PubMed] [Google Scholar]
- Zuber H. Reinigung und Eigenschaften der Carboxypeptidase aus Citrusfrüchten. Hoppe Seylers Z Physiol Chem. 1968 Oct;349(10):1337–1352. [PubMed] [Google Scholar]