Abstract
Extracellular vesicles (EVs) are not only involved in cell-to-cell communications but have other functions as “garbage bags”, as bringing nutrients to cells, and as inducing mineral during bone formation and ectopic calcification. These minuscule entities significantly contribute to the regulation of bodily functions. However, the clinical application of EVs faces challenges due to limited production yield and targeting efficiency. In our study, we propose a method for efficiently harvesting EVs utilizing simian virus 40 large T antigen (SV40LT) immortalized human placental chorionic mesenchymal stromal cells (CMSCs). We investigated immortalized placental chorionic mesenchymal stromal cells (imCMSCs), a stromal cell line that surpasses the growth limitations of primary passage cells while retaining phenotypic characteristics and differentiation potential. This development offers the prospect of a consistent, uniform source of EVs, which is essential for regenerative medicine. Our findings indicate that the immortalization process preserves the particle size, quantity and surface marker profiles of EVs, providing a possible approach to produce high-yield EVs suitable for disease diagnosis and treatment.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-87371-3.
Keywords: PMSCs, Immortality, SV40LT, Extracellular vesicles
Subject terms: Cell biology, Molecular biology, Molecular medicine
Introduction
Extracellular vesicles (EVs) are garnering increasing attention and research as novel disease biomarkers, therapeutic agents, and drug delivery platforms1. These vesicles originate from diverse cell types and are found not only in body fluids but are also embedded in the extracellular matrix2,3. As important mediators of intercellular communication, EVs play a pivotal role in physiological activities and disease development, including immune regulation, tissue repair and tumor growth4–6. According to the classification of biosynthetic pathways, EVs can be categorized into three types: exosomes (30–150 nm), microvesicles (100–1000 nm), and apoptotic bodies (50 nm–2 μm)7. In 2018, the International Society for EVs defined ‘small extracellular vesicles (sEVs)’ as those under 200 nm, extensively studied for their roles in both physiological and pathological conditions8. EVs rely on phosphatidylserine to participate in the regulation of various physiological and pathological processes, such as cellular immune responses, vascular regeneration, inflammatory responses, and neurogenesis9.It has been demonstrated that the restricted bilayer phospholipid membranes of EVs can effectively protect intracellular cargoes (proteins, nucleic acids, metabolites, etc.) from the extracellular environment, enabling the exchange of biological information between donor and recipient cells10–12. Additionally, EVs exhibit low immunogenicity, low toxicity, good biocompatibility, and can naturally traverse biological barriers such as the blood-brain barrier, making them valuable in cell-free therapies for regenerative medicine13–15.
However, EVs still face unresolved challenges in clinical use and medical research. For instance, issues such as storage methods, dosage, and administration have not been standardized16,17. At the same time, the variability in the physical, chemical properties, and functions of EVs is not fully understood, and most isolation techniques currently in use have certain limitations18.Each type of cell secretes EVs that contain their characteristics which are entirely different from other types of cells. The characteristics of EVs and non-vesicular nanoparticles depend on the differentiation stage of the cells. One cell type secretes several types of EVs and may release also non-vesicular nanoparticles. Each isolation method may yield different types of population of EVs and non-vesicular nanoparticles from the same cell type. As research into EVs deepens, understanding their biological functions and exploring their molecular mechanisms becomes crucial. Many separation techniques have been established to optimize the isolation of EVs, but currently, there is no method to ensure that the content, purity, and biological activity of EVs are all guaranteed19,20. Efficiently scaling up isolation has been a challenge in clinical applications21,22.
The function of EVs is realized through the paracrine pathway of the parental cells, and its biological activity and quantity are closely related to the parental cells and the microenvironment in which they live23,24. Mesenchymal stromal cells (MSCs) derived EVs have been demonstrated for use as alternative MSC-based therapies in regenerative medicine25,26. However, stromal cells face growth limitations during in vitro culture, such as senescence and environmental constraints27. As the number of cell divisions and proliferation increases, cell telomerase activity decreases, and telomere length shortens. It was found that there was a significant positive correlation between the degree of cell senescence and the number of cell passages. At the culture stage, the number of apoptotic cells increased significantly from the sixth passage onwards, and after 8 passages, not only did more apoptotic cells appear, but also accompanied by senescence phenomena such as cytoplasmic vacuolization and cytosolic enlargement28. The genetic stability and proliferative differentiation ability of the parental cells are particularly important when performing high-throughput production of EVs. Therefore, exogenous modification of parental cells at the source to increase the output of EVs is particularly critical for the development of future regenerative medicine therapies29,30.
In our study, we proposed a method based on human placental chorionic mesenchymal stromal cells (CMSCs), combining the simian virus 40 large T(SV40LT) gene and resistance gene screening to establish immortalized cell lines. The simian virus 40 (SV40)gene, extracted from simian kidney cell virus, consists of two parts: the large T antigen and the small T antigen31. The large T antigen is a necessary factor for initiating cellular transformation, activating late promoter transcription, establishing and maintaining the phenotype of virus-induced cell transformation31. Currently, the SV40LT gene has become a commonly used gene for immortalizing cell construction and the transfection system is simple to operate with stable effects32. Immortalized cell lines constructed using SV40 have been widely applied in various research areas including viral infections, gene expression, drug screening, and more33,34.
Building upon the aforementioned status quo, our key strategy in harvesting EVs using immortalized chorionic mesenchymal stromal cells (imCMSCs) is to eliminate the limitation of the limited lifespan of the parental cells, ensuring a single reliable source of EVs and reducing their functional heterogeneity. We chose CMSCs as our primary research subject due to their great promise for EVs isolation models. These cells possess excellent proliferation and multidirectional differentiation ability and can be used as cell models for studying various aspects such as proliferation, senescence, immunity, differentiation, and transplantation35. This approach results in the passage of stable proliferating parental CMSCs, alleviating the impact of the cellular state on the EVs. Our findings indicate that the introduction of SV40LT alone is sufficient to extend the lifespan of in vitro cultured CMSCs, leading to the production of EVs similar to those derived from primary cells. Minimal differences are observed in particle size and antigen expression levels between the two cell types.
Materials and methods
Isolation and culture of chorionic mesenchymal stromal cells
The fetal placenta was obtained from a 27-year-old woman who had normal full-term delivery. The patient experienced uncomplicated pregnancies. Prior to sample collection, informed consent was obtained from both parents of the fetus. The protocol for human placenta collection for cell isolation was approved by the Medical Ethics Committee of the Ninety-third Hospital. All experimental procedures comply with the principles of medical ethics and the requirements of the Declaration of Helsinki.
The cell extraction procedure followed established protocols36; in brief, the placenta was retrieved postpartum, immediately immersed in Dulbecco’s modified Eagle medium (DMEM) containing 4500 mg/mL of glucose and an antibiotic solution (comprising 0.1% gentamicin, 0.2% streptomycin, and 0.12% penicillin). The placenta underwent thorough rinsing with a syringe, aspirating phosphate-buffered saline (PBS) to eliminate blood clots. Subsequently, placental villous tissue was dissected into 1–2 mm-sized fragments, and 3–5 mL of trypsin solution was added to these fragments, incubating for 30 min in a 5% CO2 37 °C incubator. Following incubation, an equal volume of medium containing 10% fetal bovine serum (FBS) and antibiotic solution was introduced to neutralize the trypsin solution. The mixture was transferred to a 50 mL centrifuge tube, allowing partially digested tissue fragments to settle for 3 min. The supernatant was carefully aspirated, and 15–20 pieces of partially digested tissue debris were placed on a tissue culture flask. Incubation at 37 °C continued for 2–3 days with the addition of an appropriate amount of medium (DMEM + 10%FBS). After 3 days, the medium was replaced with fresh medium daily to remove non-adherent cells, and the culture dish was observed for cell growth around the tissue fragments. After incubating the tissue fragments for 11–14 days, daily observation of cell proliferation was conducted under an inverted microscope. Once cells migrated from the fragment boundaries and achieved approximately 80% confluence, primary CMSCs were harvested for flow cytometry characterization and immortalization studies.
Immortalization of placental mesenchymal stromal cells
The development of a lentiviral expression vector involved utilizing the PLVX-IRES-Hygro vector as a foundation, integrating the SV40LT gene through BamHI and EcoRI cleavage sites. In this vector, the expression of the SV40LT gene is driven by the EF1 promoter and includes the hygromycin resistance gene. This vector was custom-synthesized by Quan Yang Biotechnology in Suzhou. Essential components for the experiment, namely 293T cells and packaging plasmids, were housed in our laboratory. 293T cells were co-transfected using a lentiviral triple plasmid system (pLV, psPAX2, pMD2.G), in which the dosage of each plasmid was 6.5 µg of PMD2.G Vector (Addgene, ID # 12259), 8.5 µg of psPAX2 Vector (Addgene, ID # 12260), and 10 µg of shuttle plasmid37,38. The viral supernatant was collected 48 h after the start of transfection and centrifuged at 4 °C 800 g for 5 min and filtered using a 0.45 μm filter. At this point, the viral particles in the viral solution could be used for infection of the target cells. The viral particles were calculated to be around 2.7 × 108 TU/mL by measurement. The virus packaging system was specifically designed by our laboratory and has been extensively validated through experiments, achieving a transfection efficiency of 60%. Then, primary CMSCs were seeded in 24-well plates with 50,000 cells per well. Two passages were conducted to attain the optimal growth state, and cell infection was accomplished using 400 µL of viral particles for 24 h. After 48 h, screening of the thaumatin resistance gene (Beyotime, Shanghai, China) commenced (1 µg/mL). Using uninfected CMSCs as the control group (without hygromycin resistance). Following the demise of all cells in the control group, the fully grown target cells underwent passage culture.
RNA extraction, cDNA transcription, and reverse transcription-polymerase chain reaction
Total cellular RNA was extracted using an RNA extraction kit (#R0026, Beyotime Biotechnology, Shanghai, China), and the RNA concentration was determined with a NanoDrop2000 spectrophotometer (Thermo Scientific, USA)39. In brief, when the number of cells reaches 1 × 106, aspirate the supernatant from the culture medium. Add 300 µL lysis buffer to the cells. Then add an equal volume of binding solution, gently invert to mix 3–5 times. Transfer the mixture to a purification column, centrifuge at 12,000 × g for 30 s, discard the liquid in the collection tube. Add 600 µL wash buffer I, centrifuge at 12,000 × g for 30 s, discard the liquid in the collection tube. Repeat with 600 µL wash buffer II, centrifuge at 12,000 × g for 30 s, discard the liquid in the collection tube. Repeat this step once, then centrifuge at 14,000 × g for 2 min and transfer the purification column to an elution tube. Add 30 µL elution buffer, incubate at room temperature for 2–3 min, centrifuge at 14,000 × g for 30 s to obtain cellular RNA. For cDNA synthesis, the reverse transcription reaction system was prepared according to the cDNA kit instructions (#D7168S, Beyotime Biotechnology, Shanghai, China), and 2 µL RNA, 1 µL Oligo (dT) primers were used for each reverse transcription reaction. The PCR primer sequences were SV40LT-Forward: GGCTGGAGTTGCTTGGCTACAC, SV40LT-Reverse: CTGACCTGAAGGCAAATCTCTGGGAC. The PCR cycling conditions consist of an initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s. Finally, there is a final extension step at 72 °C for 10 min. Following the PCR reaction, 6 µL of the reaction mixture is loaded into a 1% agarose gel and electrophoresed at 100 v for 40 min to confirm the RT-PCR products. The telomerase activity assay was performed according to the same method. HTERT-Forward: CTGAGTATGGCTGCGTGGTGAAC, HTERT-Reverse: GTTGAAGGTGAGACTGGGCTCTGATG. The total length of the sequence was 209 bp.
Surface Antigen Identification of immortalized chorionic mesenchymal stromal cells
Upon achieving 80-90% cell confluence in the medium, cells were detached using trypsin and prepared into a cell suspension with a quantity of 1 × 106 cells/mL. Monoclonal antibodies ( BioLegend, USA) that recognize CD90 (FITC), CD73 (APC), CD105 (PE), CD34 (APC), CD45 (FITC)(at a concentration of 5 µL/106 cells) were used for staining identification40. Add 2% final concentration of fetal bovine serum to the cell suspension and co-incubate with antibodies at room temperature in the dark for 30 min. Subsequently, the expression of surface markers was detected using flow cytometry (BD FACS Celesta, BD, USA), and the results were analyzed using FlowJo software.
Multilineage differentiation
Multilineage differentiation induction encompassing osteogenic, adipogenic, and chondrogenic differentiation of both primary and immortalized cells was conducted using the Human Associated Stem Cells Induced Differentiation Kit (Cyagen, Guangzhou, China). The induction process adhered to the instructions provided by the kit. Briefly, 1–2 mL of trypsin solution was applied to cells for a total incubation of 2 min to harvest cells in the logarithmic growth phase. Cell counting ensued, with 1 × 105 cells inoculated into 24-well plates. After 24 h, cell growth status was observed, initiating differentiation induction for osteogenesis and adipogenesis. For chondrogenic differentiation, 3 × 105 cells were taken and inoculated into 15 mL centrifuge tubes for induction.
In osteogenic induction (#HUXXC-90021, Cyagen, Guangzhou, China), cells were inoculated in 24-well plates and observed for growth. Upon reaching 70-80% cell confluence, marked as day 0, the complete medium was replaced with osteogenic induction medium. Subsequently, 1–2 mL of fresh medium was replaced every 3 days. At 7–14 days of differentiation induction, morphological changes and calcium nodule formation status were observed under the microscope. After the appearance of evident calcium nodules, a half-volume fluid exchange occurred every two days. After 21 days of vitro induction, cells were fixed with a 10% formalin solution at room temperature for 30 min. Subsequently, they were stained with Alizarin Red solution at room temperature for 5 min and observed under a microscope for the formation of calcium nodules. The kit included OriCell®Basal Medium For Cell Culture (177 mL; BLDM-03011), OriCell®Fetal Bovine Serum (Superior-Quality) (20 mL; FBSSR-01021), and OriCell®Supplement For Human BMSC Osteogenic Differentiation (3 mL; HUXMX-04021).
To induce adipogenesis (#HUXXC-90031, Cyagen, Guangzhou, China), prepare induction media according to manufacturer instructions41. Induction medium A is prepared by adding inducer A (#HUXXC-04031-a) to the basal cell medium supplemented with 10% fetal bovine serum. Induction medium B is similarly prepared with inducer B (#HUXXC-04031-b). At 80–90% cell confluence on day 0, induction with medium A begins to stimulate lipid droplet formation. After 72 h, medium B is introduced to maintain existing lipid droplets. Media A and B are alternated until day 28 of differentiation. Cells are then fixed in 10% formalin for 30 min at room temperature and stained with alizarin red O staining solution (#OILR-10001, Cyagen, Guangzhou, China) for 5 min prior to observation.
Chondrogenesis differentiation was induced by chondrocyte passage medium (#HUXXC-90041, Cyagen, Guangzhou, China) under 3-dimensional culture conditions. By utilizing suspension culture, multiple adherent cells are aggregated into spheroids, thereby facilitating three-dimensional interactions between the cells and the inductive culture medium to promote induced differentiation42,43. In brief, cells were inoculated in 15mL centrifuge tubes with 500 µL of medium, placed vertically in a 37 ℃ incubator with 5% CO2 saturated humidity. After 48 h, the tubes were flicked to suspend chondrocytes in the liquid, and fresh medium (500 µL) was replaced every 3 days. After inducing for 28 days, the size of cartilage spheres was observed daily. Cartilage spheres with a diameter of 1.5–2 mm were formed in centrifuge tubes, suitable for paraffin sectioning. Following continuous dehydration of the cartilage spheres, they were immersed in xylene for a total of 4.5 h. The cartilage spheres were embedded in paraffin (xylene : paraffin = 1:1) and then cut into slices with a thickness of 3 μm. Alcian blue staining was performed at 37 °C for 1 h for observation purposes.
Soft agar clone formation assay
Before starting the experiment, a 1.4% sucrose solution was mixed with an equal volume of culture medium to prepare a final concentration of 0.7% agarose mixture. This mixture was evenly distributed and allowed to solidify at room temperature in the wells of a 6-well plate. Cell suspensions of 3rd passage CMSCs, 50th passage imCMSCs, and A549 cells were prepared and counted under a microscope for later use. The final concentration of 0.35% agar gel mixture was prepared again by mixing 0.7% sucrose solution with the medium in equal volume, and the cell suspension and gel mixture were mixed according to the amount of 10 000 cells per well, and then the mixture was added into the 6-well plate at a rate of 1 ml per well quickly, and after the top layer of agar was solidified, the plate was put into the incubator at 37 °C, 5% CO2 for 2–3 weeks, and the liquid was changed every 7 days.
Fluorescence in situ hybridization (FISH) on cells
A total of 1 × 10⁴ cells were seeded into 24-well plates with pre-positioned cover slides and incubated for 24 h. After incubation, the cover slides were carefully removed and placed in a 2× SSC solution (Beyotime Biotechnology, Shanghai, China) for aging. Gradient dehydration was then performed using ethanol. DNA denaturation of cells was carried out in a denaturation solution preheated to 73 °C. A suitable DNA probe was selected, denatured at 73 °C for 5 min, and then transferred to a 37 °C water bath for 5 min for pre-hybridization. The denatured DNA probe was applied to the cover slide, which was then placed in a humidified box and incubated at 37 °C for 16 h to allow for hybridization. Following hybridization, the slides were washed thoroughly, and DAPI solution (Yeasen, Shanghai, China) was added to the cover glass for 15 min at room temperature in the dark. After additional washing with PBS, the slides were examined under a fluorescence microscope. DNA probe information is shown in Supplementary Table 1.
Chromosomal karyotyping
Cells in logarithmic growth phase were selected, colchicine (final concentration of 0.2 ug/ mL) was added, shaken well and incubated at 37 °C for 1–2 h. Digestion was carried out using trypsin, the cell precipitate was retained by centrifugation at 800 × g, and the precipitate was resuspended with an appropriate amount of pre-warmed isotonic solution (0.075 M KCL, preparation: 8 ml sterile water + 44.7 mg KCL), shaken gently and left in a water bath at 37 °C for 40 min. The cells were then mixed with 2 mL of fixative (methanol: glacial acetic acid = 3: 1) and left at room temperature for 10 min. Centrifuge at 500 × g. The supernatant was removed and the cell precipitate was resuspended in fresh fixative solution for 10 min, centrifuged and the procedure was repeated 3 times. The cell solution (10 µL/drop) was dropped onto a slide and baked at 75 °C for 2–3 h. The dried slides were digested in freshly prepared trypsin (0.25%) for 1 min (under strict control of time) and then stained in Giemsa’s staining solution for 5–10 min, and the results were observed under a microscope after the staining solution was washed away with running water.
Isolation of Extracellular vesicles
Extracellular vesicles (EVs) were isolated using Polyethylene Glycol 8000 (PEG, #P2139, Sigma, Germany) precipitation method44. During cell culture, serum-free medium was prepared using PLTGold® Human Platelet Lysate(#PITGold500R, Sartorius, Germany) instead of FBS to mitigate its influence on subsequent experiments. When cell confluence reached 80–90%, the culture was extended for 48 h, and the medium supernatant was collected at room temperature. Upon collection, cell culture medium containing vesicles were sequentially centrifuged at 300 × g for 10 min at room temperature, followed by centrifugation at 2,000 × g for 10 min at 4 °C to remove cell debris. The supernatant was then transferred to a new centrifuge tube and centrifuged at 10,000 × g for 30 min at 4 °C to eliminate apoptotic bodies45. The supernatant was retained, and the precipitate was discarded. The post-centrifugation supernatant was immediately used for EVs isolation, with the addition of 25 mL of 10% PEG solution per 100 mL of cell supernatant46. Gentle inversion of the centrifuge tube every 20–30 min was repeated 4–5 times to ensure thorough mixing. The mixture was incubated overnight (> 12 h) at 4 °C. The following day, the mixture was centrifuged at 4 °C 10,000 g for 40 min using a tabletop centrifuge, yielding a precipitate of EVs. After discarding the supernatant from the mixture, the precipitate was dissolved in an appropriate amount of sterile PBS to obtain a solution containing EVs.
Characterization of Extracellular vesicles
The isolated EVs from the above steps were imaged using FEI Tecnai G2 TEM (Thermofisher, USA) to compare morphological changes in the outer vesicles of the two cell lines. 20 µl fresh sample solution was prepared for morphological observation, and the following settings were applied over three cycles (each with 10 positions): Focal length 100 nm, frame rate: 20, sensitivity: 70.0, and shutter speed: 70. Additionally, EVs were characterized using Single-particle interferometric reflection imaging sensing analysis (SP-IRIS) as previously described47. According to the manufacturer’s instructions, process EVs samples using the exosome isolation assay kit (#EV-TE-TRA-C, Sigma, Germany). In brief, EVs were diluted with sample buffer at a 1:1 ratio to achieve a concentration of EVs ranging from 1 × 107 to 1 × 108/mL. Subsequently, 50 µL of the diluted sample was dropwise added to the ExoView R200 chip and incubated for 16 h at room temperature. Following incubation, 1,000 µL of incubation buffer was added to wash the chip by horizontally shaking at 300 × g for 3 min. Conduct the identical procedure thrice under identical experimental conditions. Staining solution containing antibodies against CD9 (CF488A), CD81 (CF555), CD63 (CF647) was then incubated with the chip for 1 h, shielded from light, while the chip underwent slow shaking using a shaker. Finally, the chips were sequentially washed with incubation buffer and deionized water. After complete drying, the microarrays were imaged with an ExoView R200 scanner (NanoView, Boston, MA, USA), and the data were analyzed using NanoViewer 2.8.10 software.
Statistical analysis
GraphPad Prism 8.0.1 and Image J software were utilized for plotting and analyzing the experimental data. For robust and impartial outcomes, all experiments were performed multiple times with three replications. Each experiment was carried out in triplicate or more. The data were presented as mean ± standard deviation, and intergroup comparisons were conducted using one-way analysis of variance. A significance level of P < 0.05 was considered statistically significant.
Results
Enhanced long-term stability and proliferative capacity of immortalized chorionic mesenchymal stromal cells
Scientific reports suggest that stromal cells transduced with the SV40T antigen gene hold potential for clinical disease treatments48. We successfully isolated stromal cells from placental chorionic tissues obtained from a single donor, and engineered primary CMSCs into an SV40LT immortalized placenta chorionic derived mesenchymal stromal cell (imCMSCs) line. This cell line, now referred to as imCMSCs, stably express the SV40LT antigen gene through a lentiviral system (Fig. 1A). Currently in our laboratory, we successfully propagated imCMSCs stably in an in vitro environment up to the 50th passage. Primary cells reached optimal growth after 2 passages of culture. Therefore, in the next experiments, we characterized the 3rd passage parental cells and the 50th passage immortalized cells to confirm the successful establishment of the immortalized cell line.
Agarose gel electrophoresis experiments confirmed the successful introduction of the SV40LT immortalisation gene, with high expression only observed in the corresponding immortalized cell group, but not expressed in primary mesenchymal stromal cells without lentiviral transduction (Fig. 1B). The expression verification of HTERT gene showed that the gene expression of immortalized cells was higher than that of primary cells when they proliferated to 50 passages (Fig. 1C). And the expression was the same as that of the HTERT positive control group, indicating that the introduction of SV40LT gene activated telomerase activity and ensured the length of telomerase. In vitro cultivation of CMSCs exhibited the typical morphology of MSCs, oval or spindle-shaped bodies with short projections (Passage 3, Fig. 1D). With successive passages, there was a progressive transformation in cell morphology towards a fibroblast-like phenotype characterized by elongated bodies and more pronounced projections (Passage 6, Fig. 1D).
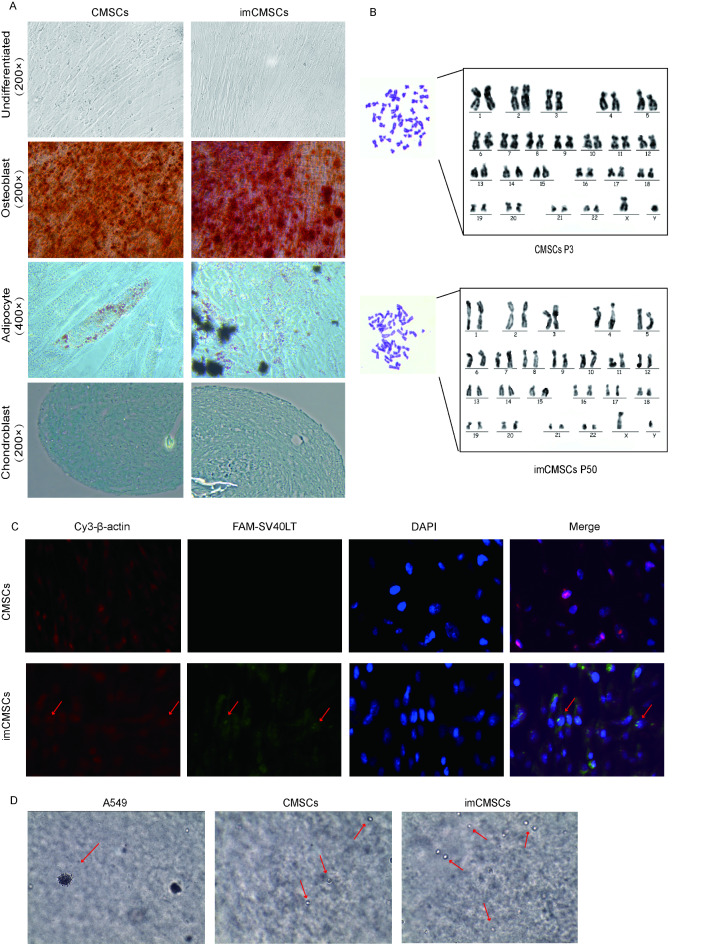
When primary CMSCs were cultured in vitro for more than 8 passages, the cells gradually transformed into a large and flat morphology and eventually entered the senescence stage (Passage 10, Fig. 1D), consistent with the results of previous studies28. In contrast, imCMSCs surpassed the senescence limits of CMSCs, exhibiting cell morphology similar to CMSCs, maintaining an adherent growth pattern (Fig. 1D). The proliferative capacity of imCMSCs surpassing the growth limits of CMSCs indicates the long-term stability potential of this cell line. Further characterization of the immortalized cell line can be achieved through subsequent experiments.
Fig. 1.
Construction and Characterization of imCMSCs. (A) Structural diagram of simian virus 40 large T antigen (SV40LT) lentiviral expression vector. (B) SV40-LT mRNA expression in placental chorionic mesenchymal stromal cells (CMSCs) and immortalized chorionic mesenchymal stromal cells (imCMSCs). M represents the marker, and the length of SV40LT is 304 bp. CMSCs at passages 3 and imCMSCs at passages 50. Technical replicates were performed, n = 3. For blots without full-length images, we have included all available images in the Supplementary Fig. 1. (C) Verification of telomerase (HTERT) gene expression by Reverse Transcription PCR(RT-PCR).M represents the marker, and the length of HTERT is 209 bp. NC is the negative control, PC is the positive control. CMSCs at passages 3 and imCMSCs at passages 50. (D) Morphological observation of CMSCs before and after SV40LT gene transduction. CMSCs at passages 3, 6, and 10 are denoted as p3, p6, and p10, respectively. imCMSCs at passages 30, 40, and 50 are denoted as p30, p40, and p50. Magnification is 100 μm. (E) Analysis of cell surface positive antigens CD90, CD73, and CD105, as well as negative markers (CD34 and CD45) by flow cytometry in P3 CMSCs and P50 imCMSCs. CD90: FITC, CD73: APC, CD105: PE, CD34: APC, CD45: FITC. n = 3.
Invariant expression of surface markers of immortalized chorionic mesenchymal stromal cells
Following the isolation and immortalization process to create imCMSCs from CMSCs, we conducted flow cytometry to analyze the expression of cell surface antigens and the absence of negative markers. The surface labelling patterns of imCMSCs closely resembled those of primary CMSCs. Both cell types displayed high expression levels of positive markers CD90, CD73, and CD105, exceeding 95% (Fig. 1E). In contrast, negative markers CD34 and CD45 were expressed at levels below 2% in both CMSCs and imCMSCs (Fig. 1E). The results showed that the introduction of SV40LT gene did not affect the expression of surface markers in MSCs.
Immortalized chorionic mesenchymal stromal cells demonstrate robust in vitro differentiation potential across multiple lineages
In vitro, different sources of MSCs can be induced to differentiate into specific cell lines in specific environments49. Therefore, we compared the in vitro differentiation potential of imCMSCs and CMSCs. The results showed that imCMSCs retained a similar differentiation capacity as 3rd passage CMSCs even after proliferation to 50 passages. Continuous induction in vitro for 21 days using osteogenic induction solution showed significant mineralised tissue formation, which was confirmed by alizarin red staining (Fig. 2A). After 21 days of induction, oil red O staining was positive, indicating the presence of lipid droplets in the cytoplasm (Fig. 2A). Chondrogenic differentiation was induced for 28 days, followed by staining with Alcian blue stain, and the cell pellets were all stained blue, demonstrating chondrogenesis (Fig. 2A). These results confirm that imCMSCs meet the basic definition of MSCs proposed by the International Society for Cellular Therapeutics (ISCT)40.
Fig. 2.
(A) Comparison of Differentiation Potential of imCMSCs. According to the International Society for Cellular Therapeutics (ISCT) definition of mesenchymal stromal cells (MSCs), the osteogenic, adipogenic, and chondrogenic differentiation abilities of immortalized chorionic mesenchymal stromal cells (imCMSCs) were assessed. The cells underwent differentiation into osteoblasts (×200), adipocytes (×400), and chondroblasts (×200) using alizarin red dye, oil red O dye, and Alcian blue dye, respectively. n = 3. (B) Comparison of karyotypic profiles between the two cell lines. (C) Fluorescence in situ hybridization (FISH) on cells (×400). Red fluorescence is β-actin gene, green fluorescence is the target gene. (D) Soft agar cloning experiments were performed to verify the in vitro safety of imCMSCs. A549 was used as a positive control and 3rd-passage CMSCs as a negative control. Magnification is 100 μm. Red arrows are cells.
Immortalized chorionic mesenchymal stromal cells exhibit Genetic Stability
When stem cells are cultured in vitro, successive passages and specific culture conditions can impact their genetic stability, potentially leading to the emergence of abnormal karyotypes50,51. To assess the genetic stability of cells associated with the SV40LT gene, we conducted gene mapping and comparative karyotype analysis of 50th passages of imCMSCs against those of the CMSCs. The results demonstrated that both cell lines maintained normal karyotypes without any chromosomal abnormalities. Furthermore, both were confirmed to be diploid, containing 46 chromosomes. The sex chromosome composition was identified as XY, indicating a male genotype (Fig. 2B). Intracellular FISH clearly revealed the insertion of the SV40LT gene in the cell’s chromosome (Fig. 2C). These findings suggest that the incorporation of the SV40LT gene does not induce chromosomal aberrations, thereby confirming the genetic stability of the imCMSCs cell line.
Immortalized chorionic mesenchymal stromal cells demonstrate Safe Stability
Using oncogenes such as SV40LT for immortalizing CMSCs may alter the safety profile of both parental cells and their secretions, potentially endowing cells and their secreted EVs with tumorigenic potential52,53. We conducted a soft agar colony formation assay to assess the tumorigenic potential of imCMSCs in vitro, using tumor cells’ ability to form colonies in semi-solid culture medium as an experimental readout. Results indicated that after 3 weeks, A549 cells as a positive control exhibited significant colony formation, whereas both 3rd-passage CMSCs and 50th-passage imCMSCs showed no colony formation (Fig. 2D). These findings suggest that both parental and immortalized cells maintained their safety profile in vitro without acquiring tumorigenic capabilities.
No change in the number of extracellular vesicles in immortalized cells
We postulated that the introduction of exogenous genes to immortalize parental cells would not alter the number of derived EVs. To test this, we isolated EVs from cell culture medium supernatants of CMSCs and imCMSCs. Subsequently, we analyzed the expression of protein markers in these EVs using SP-IRIS, focusing on the EVs tetraspanin proteins CD63 and CD81, as well as CD9, with MIgG serving as a control. In comparison to the controls, the EVs derived from imCMSCs exhibited high expression levels of CD63, CD81, and CD9. Under conditions of equal cell seeding density, the total number of EVs isolated from the conditioned media per milliliter showed no significant change between the both cell lines (Fig. 3B). However, when analyzing the number of EVs captured by individual antibody channels, we observed that CD63, CD81 and CD9 showed some variations (Fig. 3C). We speculate that these differences might be due to changes in the cells’ endosomal pathway as a result of immortalization54. Or due to the effects of the immortalization process on certain cellular functions. Morphological observation of collected EVs from both imCMSCs and CMSCs using transmission electron microscopy (TEM) revealed that EVs from both cell lines retained the characteristic cup-shaped morphology (Fig. 3A). Further examination of the particle size of EVs obtained from CMSCs and imCMSCs revealed that the peak particle size of individually captured antibodies in EVs derived from CMSCs was below 100 nm, with an average particle size of less than 85 nm (Fig. 3D). The individual peaks of imCMSCs displayed a similar pattern, with both indicating an average particle size of less than 90 nm (Fig. 3D). This finding suggests that the majority of exosomes in the isolated EVs were preserved.
Fig. 3.
No Change in the Number of Extracellular Vesicles Produced by CMSCs and imCMSCs. (A) The morphology of extracellular vesicles (EVs) secreted by chorionic mesenchymal stromal cells (CMSCs) and immortalized chorionic mesenchymal stromal cells (imCMSCs) was revealed by transmission electron microscopy (TEM). The scale is 100 nm, and the red arrow indicates exosomes. (B) Analysis of the total number of EV-tagged subpopulations in the supernatant per milliliter of culture medium produced by CMSCs and imCMSCs using a single-particle interferometric reflection imaging sensor (SP-IRIS). n = 3. Data are expressed as mean ± standard deviation. (C) Analysis of the number of CD63, CD81, and CD9-specific labelled EVs subpopulations produced by CMSCs and imCMSCs, respectively. (D) Detection of EVs particle size distribution by the SP-IRIS system. n = 3. Data are expressed as mean ± standard deviation. CMSCs-EVs: EVs isolated from the cell supernatant of primary cells cultured to the 3rd passage. imCMSCs-EVs: EVs isolated from cell supernatants after immortalizing cultured cells up to 50 passages.
No significant change in exosome number
CD63, CD81, and CD9, as four transmembrane proteins, play crucial roles in biogenesis, cargo selection, and transport, and are also signature proteins of exosomes55. Cells can influence the kinetics of exosome release by altering the release of vesicle populations56. Therefore, we employed the ExoView platform to detect the content of different vesicle populations in the samples (Fig. 4A) and determine the number of exosomes per milliliter in the samples (Fig. 4C). The mean percentages of CD63 and CD81 vesicle populations in primary cells were 13.98% and 16.04%, respectively. The number of vesicle populations increased to 16% and 17.71% after immortalization with the SV40LT gene, and the percentages of the two vesicle populations increased by 2.02% and 1.67%, respectively. In contrast, the percentage of the CD9 vesicle population decreased by 0.08%. However, none of the results were significantly different (Fig. 4B). The exosome analysis results indicate that the total number of exosomes per milliliter of cell culture supernatant remains consistent across both cell lines (Fig. 4C).Immunolocalization imaging better demonstrated the results of vesicle population secretion (Fig. 4D). These results demonstrate that the immortalization gene inserted in parental cells did not have a large effect on EVs and exosome secretion.
Fig. 4.
No change in the total number of exosomes in the two cell lines. (A) Proportion of CD63, CD81 and CD9 vesicle subpopulations to the total number of specifically labeled vesicles. CMSCs-EVs: EVs isolated from the cell supernatant of primary cells cultured to the 3rd passage. imCMSCs-EVs: EVs isolated from cell supernatants after immortalizing cultured cells up to 50 passages. (B) Mean proportions of CD63, CD81 and CD9 vesicle subpopulations in the two cell lines. Naming as above. n = 3. Data are expressed as mean ± standard deviation. (C) Total number of exosomes in the supernatant per milliliter produced by chorionic mesenchymal stromal cells (CMSCs) and immortalized chorionic mesenchymal stromal cells (imCMSCs). n = 3. Data are expressed as mean ± standard deviation. (D) Single particle interference reflection imaging sensor (SP-IRIS) fluorescence imaging was used to display the distribution of each vesicle subpopulation in CMSCs-EVs and imCMSCs-EVs. MIgG as control group.
Discussion
In this study, we demonstrate that lentiviral vectors can facilitate the stable expression of the SV40LT in CMSCs, leading to the establishment of imCMSCs line. The 50th passage of imCMSCs and their secreted EVs exhibited phenotypes and characteristics similar to those of the primary cells and their EVs.
Similar to other stromal cell sources, CMSCs have a limited proliferative lifespan in vitro culture, with senescence and apoptosis typically onset after 10 consecutive passages. In our experiments, the immortalized cell line induced by the SV40LT gene can surpass this threshold, maintaining stable proliferation for up to 50 passages under the same conditions. When using CMSCs-derived EVs for clinical research and scaled-up production, the yield of EVs can only be maintained by constantly replacing the parental cells. Variability among cell batches not only impacts the quality and consistency of EVs products but also leads to significant expenses due to repetitive testing. To address this challenge, immortalized cell lines capable of unlimited proliferation offer a solution, providing a reliable cell source for consistent and cost-effective production of EVs.
Replicative senescence occurs in all human cells cultured in vitro under specific medium conditions, resulting in irreversible cell cycle arrest in the G1 phase57,58. Immortalization occurs when SV40LT-transduced cells bypass cell cycle arrest by deactivating p53 and pRb, advancing into the S phase and altering telomerase genetics to maintain its length, thus achieving immortalization and preserving cellular function59. Presently, the overexpression of SV40LT has emerged as an efficient approach for generating immortalized cell lines60. Consequently, we utilized lentiviral vectors to develop cell lines that overexpress SV40LT, establishing imCMSCs. Our findings illustrate that solely introducing the SV40LT gene enhances the proliferative capacity of CMSCs, while the immortalized cell line retains a similar profile of stromal cell surface markers and the potential for multidirectional differentiation as the CMSCs. However, the potential tumorigenicity of immortalized cells is also a safety concern that needs to be considered. In our study, the imCMSCs not only exhibited enhanced telomerase activity but also did not have pro-tumorigenic potential in the in vitro environment, providing additional benefits.
To investigate the impact of SV40LT introduction on cell-derived EVs, we analyzed the characteristics of EVs obtained from 50th passage imCMSCs and 3rd passage CMSCs. We found that in contrast to primary cell-derived EVs, immortalized cell line-derived EVs had similar morphology, number and protein markers. Recent research has highlighted that EVs encompass various subpopulations, with distinct roles potentially influencing recipient cells differently61–63. Simultaneously, prolonged in vitro passaging cultures may alter the attributes of the resulting EVs64. Consequently, we conducted a analysis on the concentration and content of EVs subpopulations isolated from the cell culture medium supernatant. Comparing these subpopulations with those from parental MSCs, we observed some variations in the CD63, CD81, and CD9 vesicle subpopulations in imCMSCs, but not significant differences.CD63 and CD81, as components of the tetraspanins, are prominently expressed in EVs65. CD63 is known to play a role in cellular signaling pathways, facilitating EVs secretion and bolstering their biosynthetic effects66,67. Changes in CD9 expression may correlate with the type of exosomes released by the cells. Subsequently, we compared the features of exosomes secreted by both cell lines and found that the total number of exosomes per unit of medium supernatant originating from both cell lines remained stable. These results indicate that immortalization of CMSCs by the SV40LT gene does not have a major effect on their secretion of EVs and exosomes, and therefore imCMSCs can be used as a high-quality single-cell source for the production of EVs.
However, our study primarily focused on assessing the number and proportion of vesicle subpopulations. To gain insight into the effects of the immortalization process generation on specific subpopulations of EVs involved in intercellular communication, it is necessary to further characterize the main cargoes (proteins, RNAs, microRNAs) in the subpopulations of EVs. This is because the cargoes loaded by EVs change with the mode of biogenesis, parental cell type, and other factors. Human platelet lysate (HPL), when used as a supplement in cell culture media, may contain high levels of endogenous EVs, which could interfere with the purity of EVs preparations and potentially obscure the true production of imCMSCs-derived EVs. In this experiment, this issue could not be avoided, but we have acknowledged the problem. To further improve the reliability and generalizability of the results, future experiments should consider using EVs-depleted supplements, serum-free conditions, or development of new Chemical define medium (CDM) to reduce the potential impact of exogenous EVs on the outcomes. In addition, because EVs contain oncogenic mutant DNA, although imCMSCs have demonstrated non-tumorigenicity in an in vitro environment, the long-term safety of the EVs in humans and animal models requires further investigation52. In order to develop a method for large scale production of EVs, it is particularly crucial to reveal specific subpopulations of EVs-mediated crosstalk.
Comparing EVs from immortalized cell lines with primary cell lines, the immortalization of parental cells emerges as a promising approach, providing a singular source of reliable and continuous EVs while avoiding potential variability caused by the limited lifespan of parental cells and the heterogeneity between different cell sources. It is also crucial to note that additional exploration of suitable isolation methods, such as integrating size-based isolation and specific capture into a unified system, could facilitate the efficient isolation of vesicle subpopulations from EVs. In conclusion, we present a method for immortalized cell harvesting of EVs that is capable of replacing CMSCs as a high-quality cellular source of EVs.
Conclusion
This study proposes a method for obtaining EVs using immortalized stromal cells. With strong proliferative capacity persistent cell stemness, and stable cell morphology, imCMSCs provide a high-quality single cell source for EVs production and are able to eliminate heterogeneity among different cell sources. imCMSCs retained the functional properties of their parental cells, with enhanced telomerase activity and did not show tumorigenicity in an in vitro setting, and could be an ideal cell base for production development. EVs analysis showed no significant difference in the total number of vesicles suggesting the possibility of source modification of cells for sustained production of EVs. A single reliable source of EVs production is of great significance for both EVs research and EVs-related therapeutics, and we believe that our attempts will be of great help to future EVs research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Y.Y. T: Investigation, Conceptualization, Formal analysis, Data curation, Visualization, Roles/Writing - original draft, Writing – review & editing.J.S: Formal analysis, Data curation, Software, Writing – review & editing. X.J: Visualization, Data curation, Software, Writing – review & editing. X.J: Validation, Visualization, Data curation, Writing – review & editing. H.M.X: Validation, Writing – review & editing. H.W:Formal analysis, review & editing. G.H.Y: Concep-tualization, Methodology, Visualization, Writing – original draft, Writing – review & editing, Supervision, Project administration, Resources, Funding acquisition.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fonseka, P., Chitti, S. V., Sanwlani, R. & Mathivanan, S. Sulfisoxazole does not inhibit the secretion of small extracellular vesicles. Nat. Commun.12, 977. 10.1038/s41467-021-21074-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowal, J., Tkach, M. & Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell. Biol.29, 116–125. 10.1016/j.ceb.2014.05.004 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Jella, K. K. et al. Exosomes, their biogenesis and role in inter-cellular communication, tumor microenvironment and cancer immunotherapy. Vaccines-Basel6(4), 69. https://doi.org/10.3390/vaccines6040069 (2018). [DOI] [PMC free article] [PubMed]
- 4.Colombo, M., Raposo, G. & Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell. Dev. Bi. 30, 255–289. 10.1146/annurev-cellbio-101512-122326 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Barteneva, N. S., Maltsev, N. & Vorobjev, I. A. Microvesicles and intercellular communication in the context of parasitism. Front. Cell. Infect. Microbes3, 49. https://doi.org/10.3389/fcimb.2013.00049 (2013). [DOI] [PMC free article] [PubMed]
- 6.Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell. Biol.9, 654–659. 10.1038/ncb1596 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Meng, L. A., Song, K. D., Li, S. L. & Kang, Y. Exosomes: small vesicles with important roles in the development, metastasis and treatment of breast Cancer. Membranes-Basel,12(8),775. https://doi.org/10.3390/membranes12080775 (2022). [DOI] [PMC free article] [PubMed]
- 8.Théry, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles7, 1535750. https://doi.org/10.1080/20013078.2018.1535750(2018). [DOI] [PMC free article] [PubMed]
- 9.Aheget, H. et al. Exosome: a new player in translational nanomedicine. J. Clin. Med.9. 10.3390/jcm9082380 (2020). [DOI] [PMC free article] [PubMed]
- 10.Henderson, M. C. & Azorsa, D. O. The genomic and proteomic content of cancer cell-derived exosomes. Front. Oncol.2, 38. 10.3389/fonc.2012.00038 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milane, L., Singh, A., Mattheolabakis, G., Suresh, M. & Amiji, M. M. Exosome mediated communication within the tumor microenvironment. J. Control Release. 219, 278–294. 10.1016/j.jconrel.2015.06.029 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Alzhrani, G. N. et al. Exosomes: isolation, characterization, and biomedical applications. Cell. Biol. Int.45, 1807–1831. 10.1002/cbin.11620 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Gudbergsson, J. M., Jonsson, K., Simonsen, J. B. & Johnsen, K. B. Systematic review of targeted extracellular vesicles for drug delivery - considerations on methodological and biological heterogeneity. J. Control Release306, 108–120. 10.1016/j.jconrel.2019.06.006 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Tang, K. et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat. Commun.3, 1282. 10.1038/ncomms2282 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Gao, Y. et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat. Biomed. Eng.4, 743–753. 10.1038/s41551-020-0583-0 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Gelibter, S. et al. The impact of storage on extracellular vesicles: a systematic study. J. Extracell. Vesicles11, e12162. 10.1002/jev2.12162 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta, D., Zickler, A. M. & El Andaloussi, S. Dosing extracellular vesicles. Adv. Drug Deliv. Rev.178, 113961. 10.1016/j.addr.2021.113961 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Azkargorta, M. et al. Human serum extracellular vesicle proteomic profile depends on the enrichment method employed. Int. J. Mol. Sci.2210.3390/ijms222011144 (2021). [DOI] [PMC free article] [PubMed]
- 19.Ludwig, N., Whiteside, T. L. & Reichert, T. E. Challenges in exosome isolation and analysis in health and disease. Int. J. Mol. Sci.2010.3390/ijms20194684 (2019). [DOI] [PMC free article] [PubMed]
- 20.Street, J. M., Koritzinsky, E. H., Glispie, D. M. & Yuen, P. S. T. Urine exosome isolation and characterization. Methods Mol. Biol.1641, 413–423. 10.1007/978-1-4939-7172-5_23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saad, M. G., Beyenal, H. & Dong, W. J. Exosomes as powerful engines in Cancer: isolation, characterization and detection techniques. Biosens. (Basel). 10.3390/bios11120518 (2021). [DOI] [PMC free article] [PubMed]
- 22.Kimiz-Gebologlu, I. & Oncel, S. S. Exosomes, large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control Release. 347, 533–543. 10.1016/j.jconrel.2022.05.027 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Lin, Y., Zhu, W. & Chen, X. The involving progress of MSCs based therapy in atherosclerosis. Stem Cell. Res. Ther.11, 216. 10.1186/s13287-020-01728-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, J., Ge, Z., Ji, W., Yuan, N. & Wang, K. The Proosteogenic and proangiogenic effects of small extracellular vesicles derived from bone marrow mesenchymal stem cells are attenuated in steroid-induced osteonecrosis of the femoral head. Biomed. Res. Int. 4176926. 10.1155/2020/4176926 (2020). [DOI] [PMC free article] [PubMed]
- 25.Rani, S., Ryan, A. E., Griffin, M. D. & Ritter, T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol. Ther.23, 812–823. 10.1038/mt.2015.44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeo, R. W. et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv Rev.65, 336–341. 10.1016/j.addr.2012.07.001 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Mikhael, S., Beeravolu, N. & Chaudhry, G. R. Umbilical cord derivatives for intervertebral disc regeneration: advances and challenges. Cell. Gene Therapy Insights. 2, 629–634. 10.18609/cgti.2016.076 (2016). [Google Scholar]
- 28.He, Q., Ye, Z., Zhou, Y. & Tan, W. S. Comparative study of mesenchymal stem cells from rat bone marrow and adipose tissue. Turk. J. Biol.42, 477–489. 10.3906/biy-1802-52 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, H. J. et al. Changes in PTTG1 by human TERT gene expression modulate the self-renewal of placenta-derived mesenchymal stem cells. Cell Tissue Res.357, 145–157. 10.1007/s00441-014-1874-0 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Qin, S. Q. et al. Establishment and characterization of fetal and maternal mesenchymal stem/stromal cell lines from the human term placenta. Placenta39, 134–146. 10.1016/j.placenta.2016.01.018 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Ahuja, D., Saenz-Robles, M. T. & Pipas, J. M. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene24, 7729–7745. 10.1038/sj.onc.1209046 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Anand, A. P. & Gowri Sankar, A. Kokila Vani, V. Immortalization of neuronal progenitors using SV40 large T antigen and differentiation towards dopaminergic neurons. J. Cell. Mol. Med.16, 2592–2610. 10.1111/j.1582-4934.2012.01607.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshioka, M., Takenouchi, T., Kitani, H., Okada, H. & Yamanaka, N. Establishment of SV40 large T antigen-immortalized bovine liver sinusoidal cell lines and their immunological responses to deoxynivalenol and lipopolysaccharide. Cell. Biol. Int.40, 1372–1379. 10.1002/cbin.10682 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Hosoya, K., Hori, S., Ohtsuki, S. & Terasaki, T. A new in vitro model for blood-cerebrospinal fluid barrier transport studies: an immortalized choroid plexus epithelial cell line derived from the tsA58 SV40 large T-antigen gene transgenic rat. Adv. Drug Deliv. Rev.56, 1875–1885. 10.1016/j.addr.2004.07.013 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Biswas, A. et al. Human placenta/umbilical cord derivatives in regenerative medicine - prospects and challenges. Biomater. Sci.11, 4789–4821. 10.1039/d2bm01977a (2023). [DOI] [PubMed] [Google Scholar]
- 36.Beeravolu, N. et al. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J. Vis. Exp.10.3791/55224 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakuma, T., Barry, M. A. & Ikeda, Y. Lentiviral vectors: basic to translational. Biochem. J.443, 603–618. 10.1042/bj20120146 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Miller, M. R. & Blystone, S. D. Reliable and inexpensive expression of large, tagged, exogenous proteins in murine bone marrow-derived macrophages using a second generation lentiviral system. J. Biol. Methods. 10.14440/jbm.2015.66 (2015). [DOI] [PMC free article] [PubMed]
- 39.Zhao, Y. et al. Cortistatin protects against intervertebral disc degeneration through targeting mitochondrial ROS-dependent NLRP3 inflammasome activation. Theranostics10, 7015–7033. 10.7150/thno.45359 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy8, 315–317. 10.1080/14653240600855905 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Yu, F., Li, F., Yu, P., Zhou, B. & Ye, L. Identification and characterization of NFATc1(+) skeletal stem cells in bone regeneration. Cell. Rep.41, 111599. 10.1016/j.celrep.2022.111599 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Baraniak, P. R. & McDevitt, T. C. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell. Tissue Res.347, 701–711. 10.1007/s00441-011-1215-5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, Y. et al. Spheroid formation of Hepatocarcinoma Cells in Microwells: experiments and Monte Carlo Simulations. PLoS ONE11, e0161915. 10.1371/journal.pone.0161915 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Romero, N. et al. Polyethylene glycol improves current methods for circulating extracellular vesicle-derived DNA isolation. J. Transl Med.10.1186/s12967-019-1825-3 (2019). [DOI] [PMC free article] [PubMed]
- 45.Soares Martins, T. et al. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE13, e0198820. 10.1371/journal.pone.0198820 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garg, P. K. et al. Cholesterol mass efflux capacity and risk of peripheral artery disease: the multi-ethnic study of atherosclerosis. Atherosclerosis297, 81–86. 10.1016/j.atherosclerosis.2020.02.007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arab, T. et al. Characterization of extracellular vesicles and synthetic nanoparticles with four orthogonal single-particle analysis platforms. J. Extracell. Vesicles. 10, e12079. 10.1002/jev2.12079 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayashi, N. et al. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science287, 1258–1262. 10.1126/science.287.5456.1258 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Khan, W. S. & Hardingham, T. E. Mesenchymal stem cells, sources of cells and differentiation potential. J. Stem Cells. 7, 75–85 (2012). [PubMed] [Google Scholar]
- 50.Bai, Q. et al. Temporal analysis of genome alterations Induced by single-cell passaging in human embryonic stem cells. Stem. Cells. Dev.24, 653–662. 10.1089/scd.2014.0292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sardesai, V. S., Shafiee, A., Fisk, N. M. & Pelekanos, R. A. Avoidance of maternal cell contamination and overgrowth in isolating fetal chorionic villi mesenchymal stem cells from Human Term Placenta. Stem Cells Transl. Med.6, 1070–1084. 10.1002/sctm.15-0327 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hur, J. Y. & Lee, K. Y. Characteristics and clinical application of Extracellular vesicle-derived DNA. Cancers. 10.3390/cancers13153827 (2021). [DOI] [PMC free article] [PubMed]
- 53.Zhang, Y. et al. Immortalized mesenchymal stem cells: a safe cell source for Cellular or Cell membrane-based treatment of Glioma. Stem Cells Int.2022, 6430565. 10.1155/2022/6430565 (2022). [DOI] [PMC free article] [PubMed]
- 54.Raiborg, C. & Stenmark, H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature458, 445–452. 10.1038/nature07961 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Kumari, S., Devi, G., Badana, A., Dasari, V. R. & Malla, R. R. CD151-A striking marker for cancer therapy. Biomark. Cancer. 7, 7–11. 10.4137/BIC.S21847 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Vlist, E. J. et al. CD4(+) T cell activation promotes the differential release of distinct populations of nanosized vesicles. J. Extracell. Vesicles. 10.3402/jev.v1i0.18364 (2012). [DOI] [PMC free article] [PubMed]
- 57.Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell. Res.37, 614–636. 10.1016/0014-4827(65)90211-9 (1965). [DOI] [PubMed] [Google Scholar]
- 58.Goldstein, S. Replicative senescence: the human fibroblast comes of age. Science249, 1129–1133. 10.1126/science.2204114 (1990). [DOI] [PubMed] [Google Scholar]
- 59.Li, P. et al. Generation of a new immortalized human lung pericyte cell line: a promising tool for human lung pericyte studies. Lab. Invest.101, 625–635. 10.1038/s41374-020-00524-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li, X. et al. Highly proliferative immortalized human Dental Pulp cells retain the Odontogenic phenotype when combined with a Beta-tricalcium phosphate Scaffold and BMP2. Stem Cells Int.2020, 4534128. 10.1155/2020/4534128 (2020). [DOI] [PMC free article] [PubMed]
- 61.Kowal, J. et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. U S A113, E968–977. 10.1073/pnas.1521230113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lasser, C. et al. Two distinct extracellular RNA signatures released by a single cell type identified by microarray and next-generation sequencing. RNA Biol.14, 58–72. 10.1080/15476286.2016.1249092 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willms, E. et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep.6, 22519. 10.1038/srep22519 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allen, M., Bjerke, M., Edlund, H., Nelander, S. & Westermark, B. Origin of the U87MG glioma cell line: good news and bad news. Sci. Transl Med.8, 354–353. 10.1126/scitranslmed.aaf6853 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Andreu, Z. & Yanez-Mo, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol.5, 442. 10.3389/fimmu.2014.00442 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dogrammatzis, C., Deschamps, T. & Kalamvoki, M. Biogenesis of extracellular vesicles during herpes simplex virus 1 infection: role of the CD63 Tetraspanin. J. Virol.93. 10.1128/JVI.01850-18 (2019). [DOI] [PMC free article] [PubMed]
- 67.Israels, S. J. & McMillan-Ward, E. M. CD63 modulates spreading and tyrosine phosphorylation of platelets on immobilized fibrinogen. Thromb. Haemost. 93, 311–318. 10.1160/TH04-08-0503 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.