Abstract
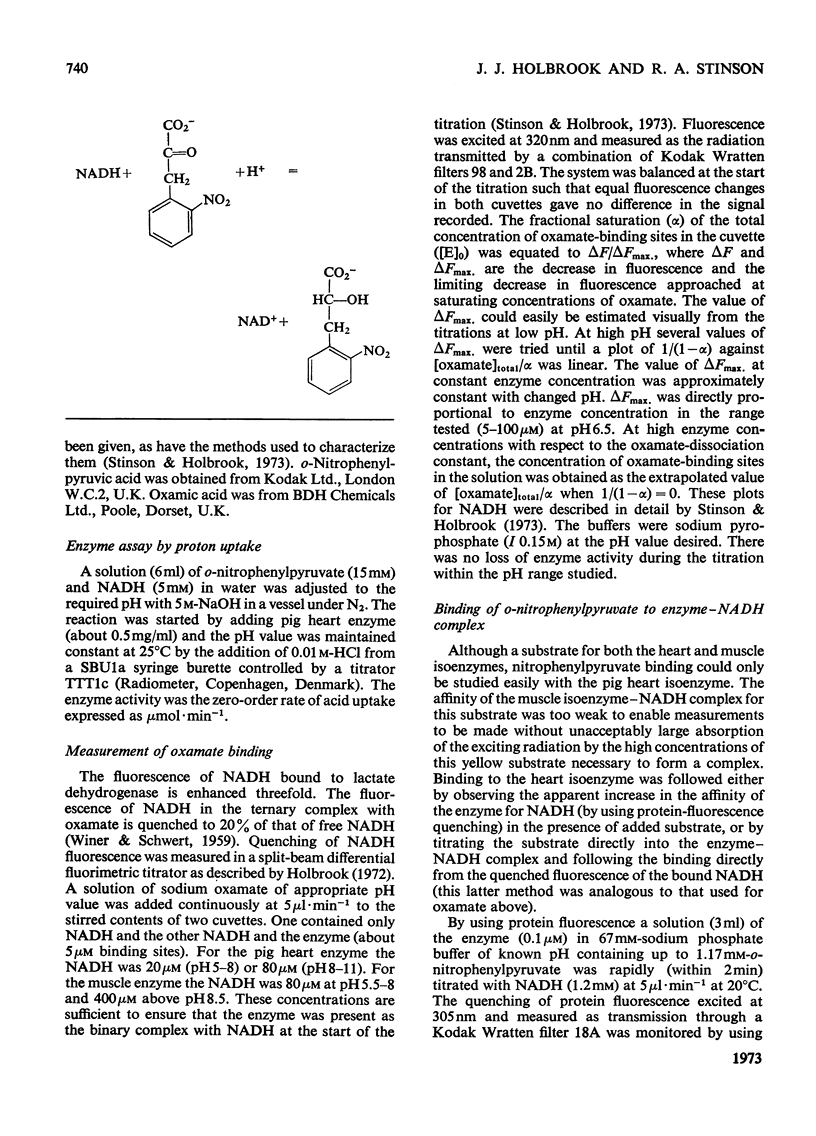
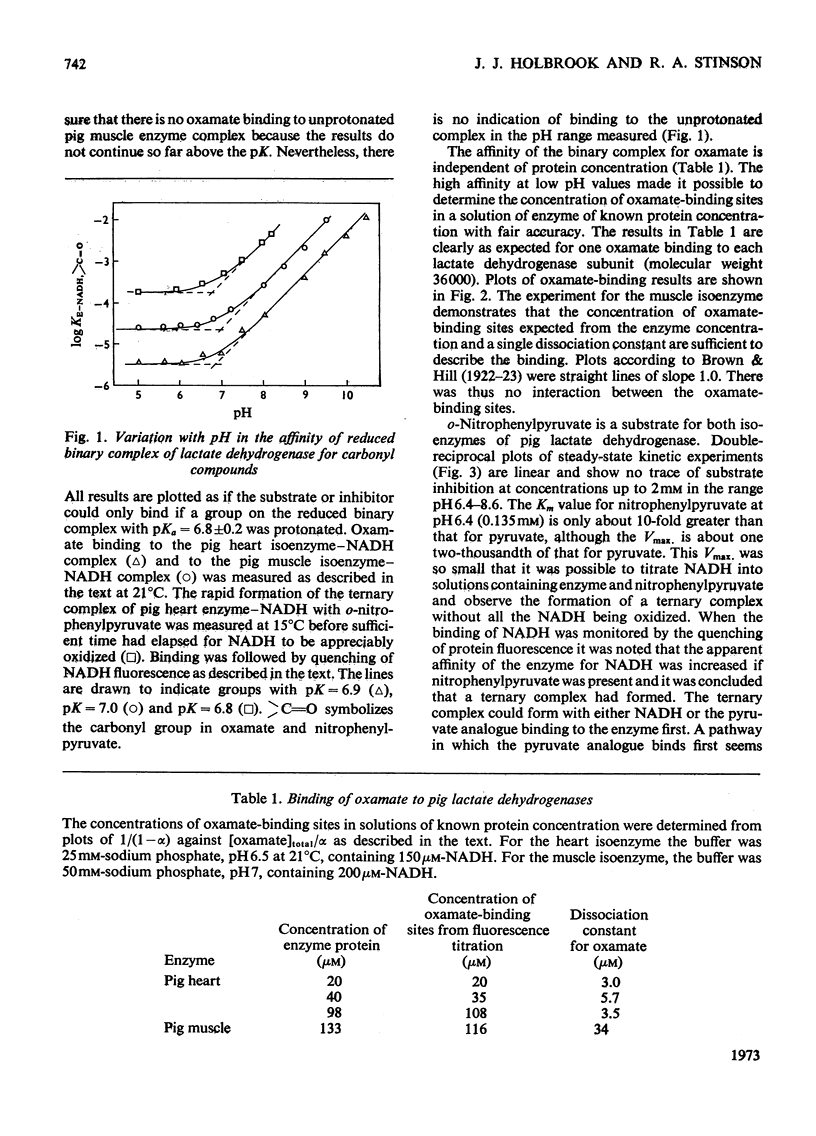
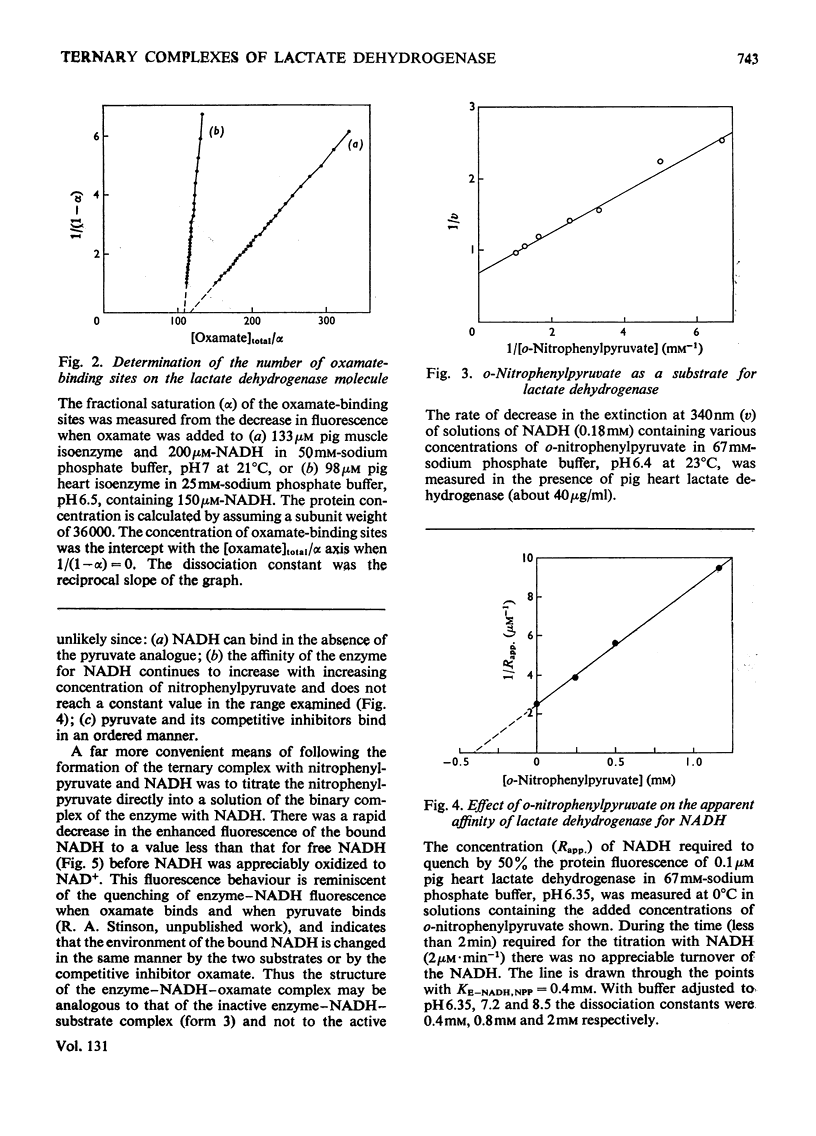
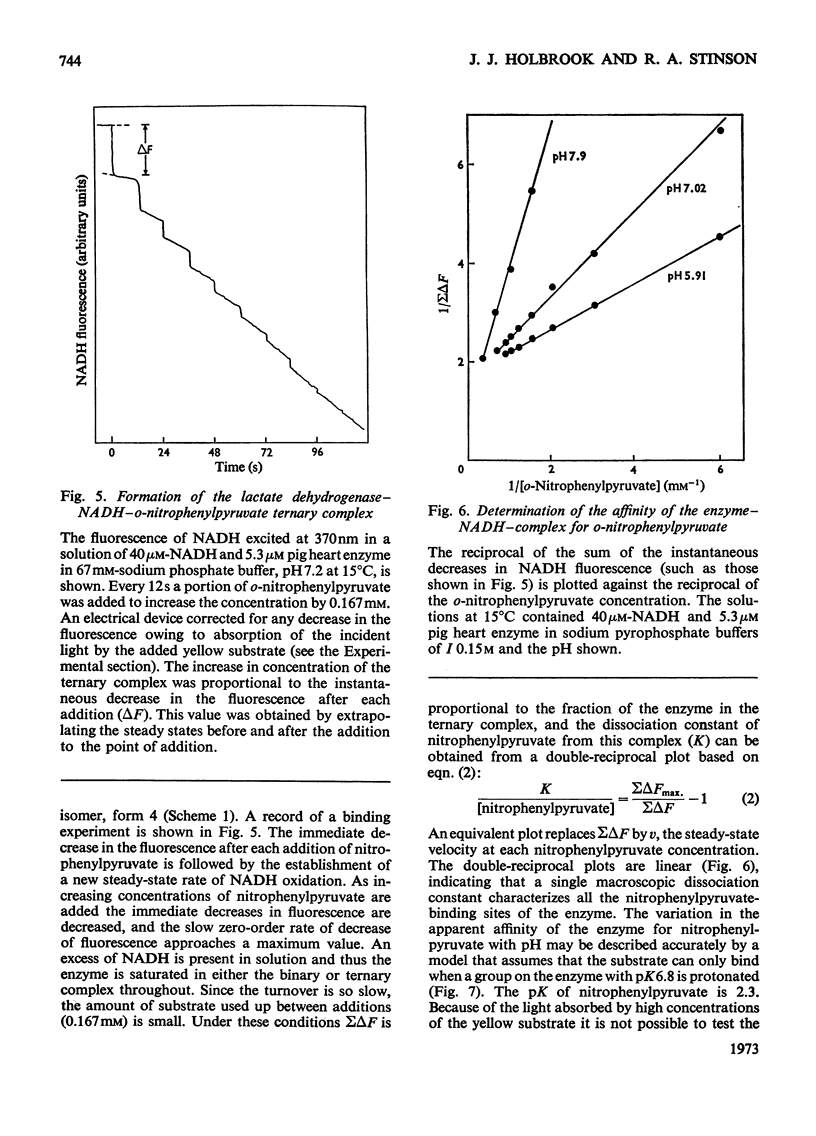
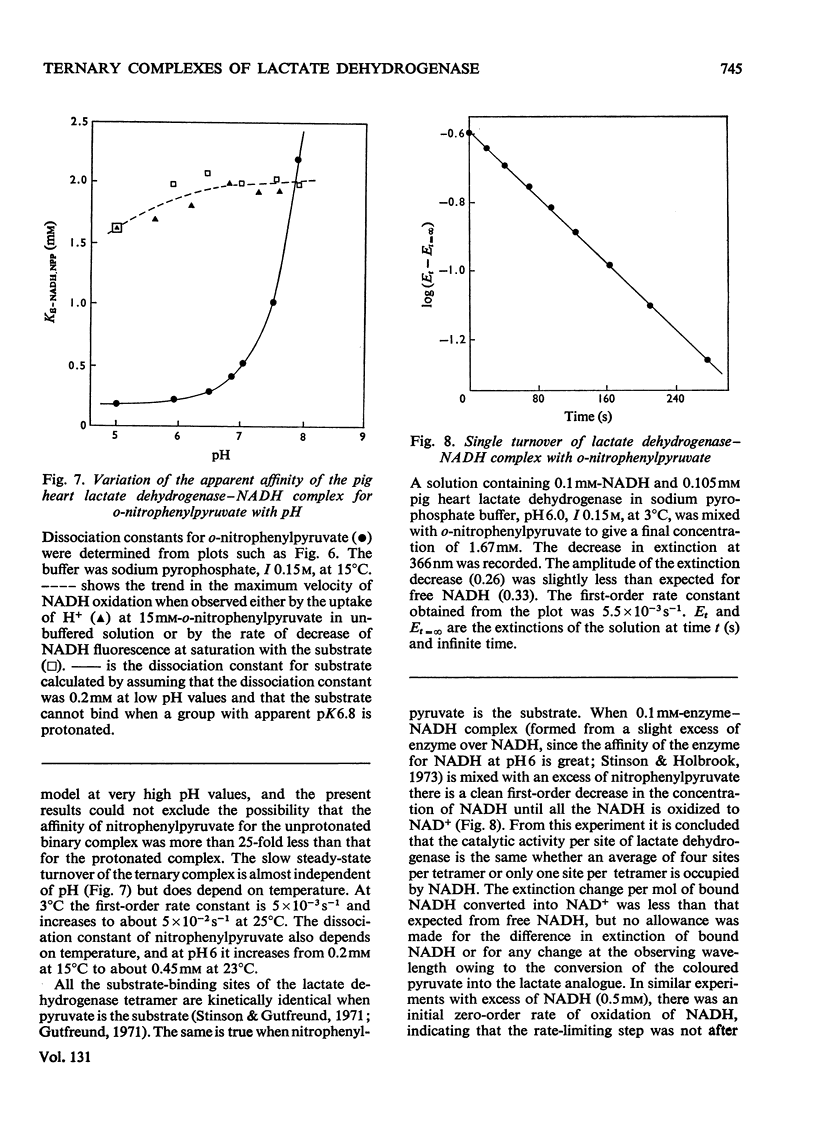
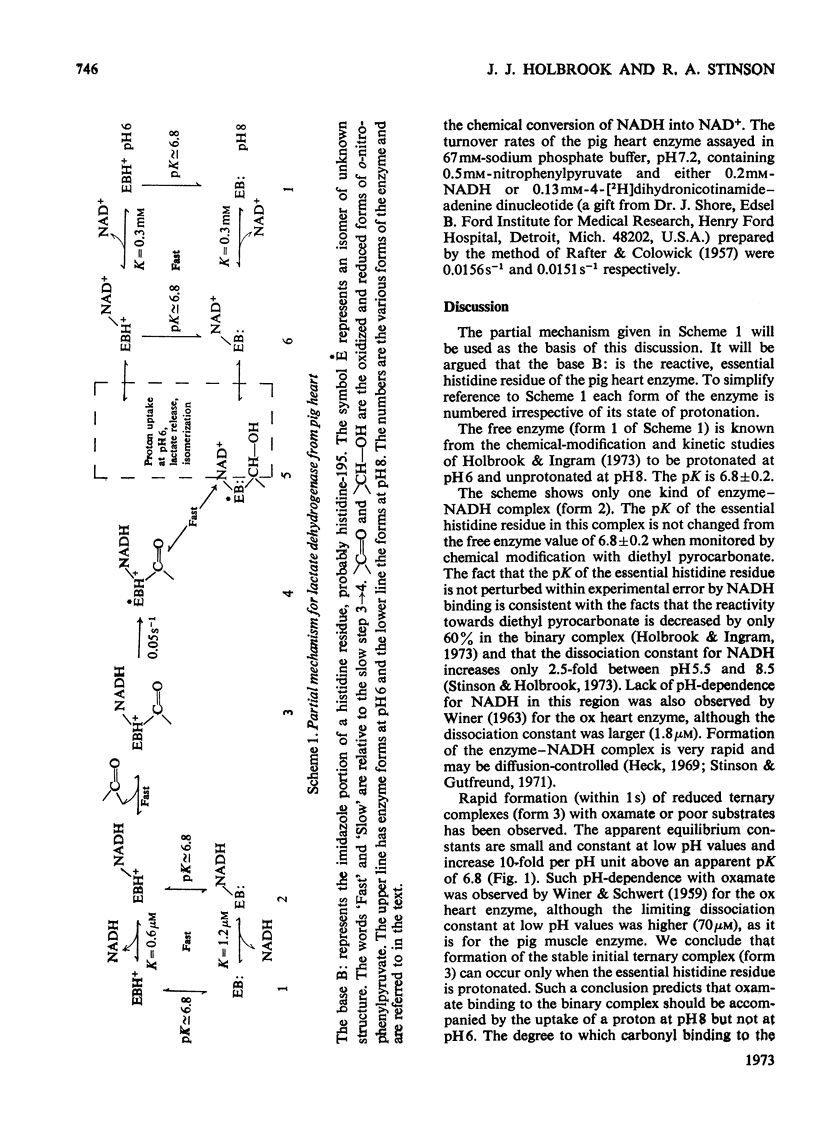
1. The binding of oxamate to pig heart and pig muscle isoenzymes of lactate dehydrogenase in the presence of NADH was studied by fluorescence titration. The dissociation constant of oxamate from the heart enzyme complex is 3μm and from the muscle isoenzyme 25μm at pH5. These values quantitatively increase with pH as predicted if oxamate can bind only to the enzyme–NADH complex if a group with pK6.9 is protonated. There are four non-interacting oxamate-binding sites per tetramer. 2. o-Nitrophenylpyruvate is a poor substrate for both isoenzymes but has a reasonable affinity to the heart isoenzyme. Initially, it forms an enzyme–NADH–substrate complex, which can be detected either by protein-fluorescence quenching or by NADH-fluorescence quenching. The pH-dependence of the dissociation constant of nitrophenylpyruvate also shows that this ternary complex can only form if a group with pK6.8 is protonated. Taken with the results of chemical-modification experiments, these results allow the pK of 6.8 to be assigned to a system probably involving the imidazole side chain of histidine-195. Formation of a ternary complex from a binary one at pH8 is predicted to result in a proton being taken up from solution. 3. Isotope-effect studies with NADH and its deuterium analogue show that the rapidly formed ternary complex with o-nitrophenylpyruvate slowly isomerizes to give an active ternary complex, which then rapidly decomposes to NAD+. The isomerization is pH-independent, and it is suggested that histidine-195 is still protonated in the activated ternary complex, which is present before hydride transfer. 4. All four subunits of the enzyme are kinetically equivalent with respect to the oxidation of bound NADH by o-nitrophenylpyruvate. 5. A partial mechanism for the enzyme is described which emphasizes the isomerizations and ionizations involved in forming the reduced ternary complex at pH6 and 8.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gutfreund H. Transients and relaxation kinetics of enzyme reactions. Annu Rev Biochem. 1971;40:315–344. doi: 10.1146/annurev.bi.40.070171.001531. [DOI] [PubMed] [Google Scholar]
- Heck H. de A. Porcine heart lactate dehydrogenase. Optical rotatory dispersion, thermodynamics, and kinetics of binding reactions. J Biol Chem. 1969 Aug 25;244(16):4375–4381. [PubMed] [Google Scholar]
- Holbrook J. J., Ingram V. A. Ionic properties of an essential histidine residue in pig heart lactate dehydrogenase. Biochem J. 1973 Apr;131(4):729–738. doi: 10.1042/bj1310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J. Protein fluorescence of lactate dehydrogenase. Biochem J. 1972 Jul;128(4):921–931. doi: 10.1042/bj1280921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Adams M. J., Buehner M., Ford G. C., Hackert M. L., Lentz P. J., Jr, McPherson A., Jr, Schevitz R. W., Smiley I. E. Structural constraints of possible mechanisms of lactate dehydrogenase as shown by high resolution studies of the apoenzyme and a variety of enzyme complexes. Cold Spring Harb Symp Quant Biol. 1972;36:179–191. doi: 10.1101/sqb.1972.036.01.025. [DOI] [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. Lactic dehydrogenase. III. Mechanism of the reaction. J Biol Chem. 1956 Nov;223(1):157–170. [PubMed] [Google Scholar]
- Schellenberg K. A. Tryptophan as intermediate in dehydrogenase action. 3. Evidence for complete cycle of hydrogen transfer between substrate and tryptophanyl residues in rabbit muscle lactate dehydrogenase. J Biol Chem. 1967 Apr 25;242(8):1815–1820. [PubMed] [Google Scholar]
- Schwert G. W., Miller B. R., Peanasky R. J. Lactic dehydrogenase. X. A re-evaluation of the effects of pH upon the kinetics of the reaction. J Biol Chem. 1967 Jul 25;242(14):3245–3252. [PubMed] [Google Scholar]
- Shore J. D., Gutfreund H. Transients in the reactions of liver alcohol dehydrogenase. Biochemistry. 1970 Nov 24;9(24):4655–4659. doi: 10.1021/bi00826a006. [DOI] [PubMed] [Google Scholar]
- Stinson R. A., Gutfreund H. Transient-kinetic studies of pig muscle lactate dehydrogenase. Biochem J. 1971 Jan;121(2):235–240. doi: 10.1042/bj1210235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson R. A., Holbrook J. J. Equilibrium binding of nicotinamide nucleotides to lactate dehydrogenases. Biochem J. 1973 Apr;131(4):719–728. doi: 10.1042/bj1310719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W. Lactic dehydrogenase. VII. Fluorescence spectra of ternary complexes of lactic dehydrogenase, reduced diphosphopyridine nucleotide, and carboxylic acids. J Biol Chem. 1959 May;234(5):1155–1161. [PubMed] [Google Scholar]
- Woenckhaus C., Berghäuser J., Pfleiderer G. Markierung essentieller Aminosäurereste der Lactat-Dehydrogenase aus Schweineherz mit (Carbonyl-14C)3-(2-Brom-acetyl)-pyridin. Hoppe Seylers Z Physiol Chem. 1969 Apr;350(4):473–483. [PubMed] [Google Scholar]


