Abstract
Introduction:
The tools to distinguish relapse from reinfection are needed in malaria-endemic areas. We evaluated seroprevalence against sets of specific peptides to the block 2 region of Plasmodium vivax-merozoite surface protein-1 (PvMSP1) to detect parasite clones.
Methods:
We applied amplicon deep sequencing (ADS) of block 2 region of the MSP-1 gene (pvmsp1) to determine cocirculating parasite clones within eight P. vivax-infected individuals. Based on this, a seroprevalence of IgM and IgG antibodies against sets of peptides of different block-2 haplotypes was validated. After, we evaluated the seroprevalence in plasma of 72 pregnant women, from which 31 had recurrent P. vivax infections.
Results:
ADS revealed one block 2 haplotype clone infecting five of eight P. vivax-infected individuals. In all, IgM antibodies, not IgG, recognized only a set of peptides specific to the block 2 haplotype determined by ADS. In the other three patients, ADS determined three concurrent block 2 haplotype clones, among whom there was always one haplotype that predominated with more than 95% of high-quality reads and two other smaller haplotypes with up to 5% and the least was <1%. We observed higher IgM levels against haplotype-specific peptides corresponding to the predominant clone. The seroprevalence of pregnant women showed that anti-haplotype-specific IgM detected coinfection with parasite clones per pregnant woman and we also observed levels of anti-haplotype-specific IgM in primary infection increased in some recurrent episodes.
Conclusion:
IgM against sets of peptides specific to different pvmsp1 haplotypes may be employed as a serological marker for parasite clones in vivax malaria.
Keywords: Anti-block 2 merozoite surface protein 1, IgM, Plasmodium vivax, pregnant woman, recurrence
INTRODUCTION
Genotyping Plasmodium vivax with microsatellite markers offers insights into parasite diversity; however, drawbacks include hypervariability and low sensitivity to detect minority alleles, limiting their utility for tracking individual genotypes and recurring variants longitudinally in endemic areas.[1] Amplicon deep sequencing (ADS) for highly polymorphic molecular markers has become an innovative tool for the identification of multiclonal infections based on unique haplotypes.[1]
Relapses in P. vivax infections often involve genetically distinct parasites from those already present in the bloodstream (referred to as multiplicity of infection [MOI]).[2,3] Hence, tools that facilitate the comprehension of the underlying population structure of P. vivax are imperative. Merozoite surface protein-1 (MSP1) is coded by a single-copy gene, making it suitable for genotyping individual P. vivax clones as its ortholog in Plasmodium falciparum infections.[3,4,5,6,7,8] Herein, we demonstrated, first, that a seroprevalence of antibodies against sets of haplotype-specific peptides to the Block 2 region of P. vivax MSP1 (PvMSP1) (block 2 peptides) is capable of confirming the infection of parasite clones determined by ADS. Given the risk of relapse of P. vivax malaria during pregnancy, we assessed whether the IgM and IgG anti-haplotype-block 2 antibodies can be used to detect parasite clones infecting pregnant women in primary infection (PI) and across subsequent recurrences during pregnancy.
METHODS
Research quality and ethics statement
This study received approval from the Institutional Review Board/Ethics Committee (Fundação de Medicina Tropical Dr. Heitor Vieira Dourado IRB# 42021515.0.3001.0005 and University of São Paulo IRB# 50158321.0.0000.5467).
Blood samples used in the amplicon deep sequencing to determine parasite clones of Plasmodium vivax-infected patients
Blood samples were obtained from eight patients diagnosed with malaria by P. vivax attended at the Tropical Medicine Foundation Dr. Heitor Vieira Dourado (FMT-HVD) in Manaus, Brazil. 10 mL of peripheral blood was collected immediately after confirmation of monoinfection in the thick blood smear by microscopy. They were identified as PV01 until PV08. DNA extraction was performed using the Qiagen genomic DNA extraction kit. A polymerase chain reaction (PCR) was conducted to generate 350–400 base pair (bp) amplicons of the block 2 region of the pvmsp1 gene according to the description.[8] The amplicons were stored at −20°C until sequencing.
Preparation of amplicon deep sequencing in ion torrent – PGM™ system
The up to 400 bp Block 2 pvmsp1 amplicon library was prepared and labeled from the 8 samples with equimolar inclusion of a multiplex identifier (barcodes) using Ion OneTouch™. At the end of the reaction, a purification step was performed using the Agencourt® AMPure® XP reagent following the manufacturer’s guidelines. Once the preparation of the library was completed, the PCR step was performed in emulsion with ion sphere particles (ISPs) containing complementary sequences to the adapter P1, using the Ion PGM™ template OT2 200 kit (Cat. 4480974-Life Technologies/Ion Torrent™) on Ion OneTouch™ System 2 (Life Technologies) equipment. The ISPs were enriched and placed on a 314 chip in the Ion PGM™ system to the sequencing that took place. The data generated were initially analyzed using the Torrent Suite 4.2 software. Raw sequence reads were separated based on the barcodes from the pooled data into amplicon-specific data, then filtered according to read length, overall quality scores, and presence of primer sequences. Sequences were edited and translated into amino acid sequences using the DNAstar package to identify haplotype sequences located at the beginning of block 2.
Block 2 Plasmodium vivax-merozoite surface protein-1 haplotypes and development of sets of block 2 peptides
The genetic diversity of P. vivax MSP1 block 2 variants found in parasites circulating in Manaus and other endemic regions of Brazil and the world.[8] A mosaic has been created using different colors to facilitate the comprehension of diversity [Supplementary Figure 1 (2.5MB, tif) ]. Amino acid sequences containing predicted B-cell epitopes were chosen for synthesis as peptides composed of 16–20 residues, corresponding to each PvMSP1-Block 2 haplotype. Peptides were synthesized in the solid phase using the Fmoc strategy (9-Fluorenil-metoxicarbonila-link), deprotected with trifluoroacetic acid/water/1,2-ethanodithiol/triisopropylsilane, and eluted in aqueous acetonitrile on reverse phase chromatography on a Sephasil® C8 peptide column in high-performance liquid chromatography [Supplementary Table].
Supplementary Table.
Block 2 peptides sets.
| Haplotype | Haplotype Sequence | Block 2 peptides |
|---|---|---|
| H 1 | DENAKRGSTQSNTTNGTGAQNNAAQGSTGNTETGTQSSA | NTTNGTGAQNNAAQG |
| QGSTGNTETGTQSSA | ||
| SSASSTNLSGGAGTT | ||
| RGGQSTNTTDGTGAQNNAA | ||
| GTTVVGTSSPAPAAP | ||
| PAAPSSTNANYEAKKI | ||
| H 2 | DENAKRGQSISISTISGNGAQTDGHQ | SISTISGNGAQTDGHQ |
| STGNRSPSQAPAAP | ||
| RSPSQAPAAPPAASS | ||
| H 3 | DENAKRGGTQSNTTNGTGAQTDGHQPTTASSET | SSETTQNSGSSGTGSSD |
| QNSGSSGTTQNSGSTGN | ||
| GNISHPAAPAASSPT | ||
| PAASSPTDENYTNKKA | ||
| H 4 | STENDKRNGGGQYTDMINGNGTQA | GGQYTDMINGNGTQA |
| TGSTGNISPSQARAD | ||
| QARADSPSTGTDYNAK | ||
| H 5 | AENKKRSGHPTT | LIIAENKKRSGHPTT |
| TTTTNGAGTQPANGSIA | ||
| IAAASSETTQISGSSN | ||
| SGSTGHGSSNPGSSGTG | ||
| STGNGQSPPATADASP | ||
| PPATADSPSTGTDYNA | ||
| H 6 | EEHKKRSGQYT | EEHKKRSGQYT |
| RSGQYTSTISGN | ||
| STGNRSSSQARA |
We designed sets of block 2 peptides containing major semi-conserved sequences upstream of repeats of six Block 2 Plasmodium vivax-merozoite surface protein-1 haplotypes, referred to as H1, H2, H3, H4, H5, and H6. In addition, B-cell epitope predictions were carried out on all peptides using six haplotypes of HLA DRB1: 0101, 0301 (DR17), 0401 (DR4Dw4), 0701, 1101, and 1501 in the BcPred algorithm (red underlined bars).[1] In ABCPred, putative epitopes with 12 amino acids in length were generated with a specificity of 75% and predicted with window length of 10 and threshold 0.7. Underlined aa refers to the suggested epitope with aa score ≥0.7 (blue underlined bars)[1]
Enzyme immunosorbent assay
The enzyme-linked immunosorbent assay (ELISA) was conducted using HRP-conjugated mouse monoclonal antibodies against human IgG (IG266) and human IgM (ICL-931) (Novus Biologicals). A cutoff value was established for each peptide using plasma samples from 10 healthy individuals who had never had malaria following the method outlined by Versiani et al.[9] The reactivity index (RI) was determined by the mean OD of the duplicates of each sample divided by the cutoff value. Samples with an RI ≥1 were considered positive.
Plasmas from Plasmodium vivax-infected pregnant women
Plasmas from 72 P. vivax-infected pregnant women were provided by Professor Dr. Claudio Marinho (ICB-USP).[10,11,12] Pregnant women infected with P. vivax were enrolled in these studies through volunteer sampling and followed up until delivery. Blood samples were collected during these visits, and additional samples were obtained for each episode of malaria during pregnancy. Malaria screening was performed by microscopy. Upon diagnosis, all pregnant women infected with P. vivax received treatment with chloroquine over a 3-day period with successful treatment confirmed by microscopy. No hypnozontocidal treatment with primaquine was administered.[13]
Of the 72 plasma samples provided, 41 had only the PI during pregnancy (labeled as samples 1–41), 17 had a PI and one recurrence episode (Group PI + 1R) in the same pregnancy (labeled as samples 42–58), and 14 women had the PI and two or more recurrent episodes during the same pregnancy (Group PI ≥2R, labeled as samples 59–72).
Data treatment and statistical analysis
The nonparametric Kruskal–Wallis test was employed to evaluate IgM and IgG levels, as well as the number of recognized peptides, among the three groups. In the case of two groups, the Mann–Whitney U-test was utilized. Statistical significance was defined as P < 0.05.
RESULTS
Amplicon deep sequencing and haplotype-specific IgM
IgM and IgG antibody responses against sets of Block 2 peptides were assessed with plasmas of P. vivax-infected patients whose parasite population was characterized on Block 2 pvmsp1 haplotypes by ADS. Two samples (PV04 and PV08) were from individuals with PIs, and ADS revealed infection by only haplotype H1 in 100% of the reads [indicated by arrows in Figure 1a]. Histograms illustrating the IgM and IgG antibody responses of both patients demonstrate the recognition of peptides from haplotype 1 by only IgM.
Figure 1.

Amplicon deep sequencing (ADS) and specific haplotype antibody response as a new tool for the exposure to haplotype block 2 variants in actual infection (a) Deep sequencing was employed to analyze polymerase chain reaction products derived from the block 2 region of the Plasmodium vivax-merozoite surface protein-1 (MSP1) gene, aiming to identify parasite clones according to MSP1 Block 2 haplotypes in two primary-infected individuals (PV04 and PV08). Arrows highlight the detection of a single clone in 100% of the reads. Both patients were infected with haplotype 1 (H1). Histograms depicting the IgM and IgG antibody responses of both patients illustrate the recognition of peptides from haplotype 1 exclusively by IgM; (b) The same strategy has been used here with 3 patients who already had a history of malaria (PV02, PV03, and PV05). Arrows highlight the detection of a single clone in 100% of the reads, who were infected by only one clone based on the block 2 haplotype of MSP1. Patients PV02 and PV05 were infected by haplotype H1 and patient PV03 by haplotype H4. Histograms depicting the IgM and IgG antibody responses. Comparison of IgG reactivity between peptides corresponding to the clone of the current infection in relation to the peptides of other haplotypes; (c) Quantification of the reads of sequences obtained in the ADS of block 2 in three patients who showed infection by 3 clones based on the haplotype of MSP1 block 2 (for more details consult materials and methods). Histograms depicting the IgM and IgG antibody responses. Comparison of IgG reactivity between haplotype-specific peptides by t-test used
The six remaining samples were obtained from individuals with a history of malaria [Figure 1b]. Three showed infection by a specific haplotype (PV02, PV03, and PV05). The haplotype H1 was detected in PV02 and PV05, whereas haplotype H4 was identified in PV03. Histograms depicting IgM and IgG antibody responses for each patient are provided. The IgM antibody response illustrates the recognition of peptides only from the haplotype detected by ADS [indicated by arrows in Figure 1b]. Regarding IgG, plasma from patient PV03 did not exhibit a switch to IgG, whereas plasma from patients PV02 and PV05 did. In addition, IgG antibodies to other haplotypes suggest previous exposure to malaria.
Samples PV01, PV06, and PV07 each showed infection by three haplotypes, with all patients having a history of four previous episodes of malaria [Figure 1c]. ADS enabled the quantification of these haplotypes, revealing a predominant haplotype with more than 95% of the reads in all samples [see frequencies of haplotypes by ADS in Figure 1c]. In the same figure, the IgM and IgG histograms illustrate antibody responses of patients PV01, PV06, and PV07 against sets of peptides corresponding to the respective haplotypes identified in ADS. In sample PV07, we identified haplotype H5 with 97.6% of the reads, followed by H1 with <2% of reads, and H4 with <0.1%. In sample PV01, the infection involved haplotypes H5, H4, and H1, in descending order. In the PV07 isolate, Mann–Whitney tests revealed statistically different IgM levels against peptides between haplotypes H5 and H1 (t-test P = 0.02). IgM levels against the predominant haplotype were statistically higher than antibody levels against H1, which was the second-most frequent but accounted for <1% of reads. Conversely, IgG levels were not correlated with the parasite load estimated by the number of reads. The IgG level against haplotype H5 was negative (below the cutoff reactivity obtained with negative control plasmas).
Sample PV01 was infected with haplotypes H5, H4, and H1 in descending order [see frequencies of haplotypes by ADS in Figure 1c]. Similarly, IgM levels against the predominant haplotype H5 were statistically higher than antibody levels against H4, which was the second-most frequent, and the least common haplotype, H1 [see IgM histogram, Figure 1c]. In this sample, IgG levels against peptides of the predominant haplotype H5 were higher than those against peptides of H4 (the minor haplotype) but did not differ from IgG levels against peptides of haplotype H1, which was the least common minor haplotype [see IgG histogram, Figure 1c].
The same was observed with the sample PV06 that was infected with the haplotypes H3, H4, and H2 in descending order [see frequencies of haplotypes by ADS in Figure 1c]. The IgM levels against the predominant H3 were statistically higher than haplotypes H4 and H2 [see IgM histogram, Figure 1c]. The IgG levels against H4 were not correlated with the parasite load estimated by the number of reads, whereas to the other haplotypes the IgG were negative [see IgG histogram, Figure 1c].
IgM anti-block 2 haplotypes of Plasmodium vivax-merozoite surface protein-1 in malaria recurrences during pregnancy
IgM and IgG antibody responses were assessed for detecting exposure and re-exposition to individual clones of the parasite in P. vivax-infected pregnant women that had recurrences during pregnancy. Out of 72 P. vivax-infected pregnant women tested, 41 had only PI during pregnancy, whereas 31 experienced recurrences during the same pregnancy, subdivided into 17 with one recurrence episode and 14 with two or more recurrent episodes. The time of PI was similar between groups; in the ONLY_PI group, the PI occurred at a median of 23 weeks of gestational age (IQ25 = 15 and IQ75 = 30), whereas the median for the PI + 1R and PI ≥2R groups was both 23 weeks (IQ25 = 15 and IQ75 = 29) and 19 weeks (IQ25 = 14 and IQ75 = 25), respectively [Figure 2a-c].
Figure 2.
Distribution of the IgM and IgG reactivity from the serum of pregnant women with vivax malaria divided into groups (a) ONLY_ primary infection (PI) group: women who have had only a single malaria infection during pregnancy (b) PI + 1R group: women who had an infection and a relapse of malaria (c) PI ≥2R group: women who had an infection and 2–4 relapses. Each line represents a pregnant woman. Sets of block 2 peptides were developed against variant sequences of six block 2 sequences of Plasmodium vivax MSP1 and referred to as H1 to H6. White square-negatives. Gray square serums with IgM reactivity against respective peptides in which they appeared only once at some point during pregnancy. Red square serums with IgG reactivity. The time of PI between groups. In the ONLY_PI group, PI occurred with a median of 23 weeks of pregnant age (IQ25 = 15 and IQ75 = 30), while the median of PI + 1R and PI ≥2R groups were 23 weeks (IQ25 = 15 and IQ75 = 29) and 19 weeks (IQ25 = 14 and IQ75 = 25)
In terms of those who experienced recurrent P. vivax infections, the mean interval for the second episode in the PI + 1R group was 8 weeks (ranging from 5 to 30 weeks), well above the half-life of IgM in plasma. This suggests an increase in IgM antipeptide levels compared to those in the PI, which may indicate recrudescence or re-exposure to the same parasite populations. As for pregnant women in the PI ≥2R group, the shortest period between the first and second episodes was 2 weeks, and the longest was 13 weeks. Between the second and third episodes, the shortest period was 1 week, whereas the longest was 11 weeks.
Figure 2a illustrates the IgM and IgG antibody responses during the PI among women in the ONLY_PI group. Figure 2b displays IgM and IgG antibody responses during the PI and recurrences among women in the PI + 1R group. Figure 2c shows IgM and IgG antibody responses during the PI and successive recurrences among women in the PI ≥2R group. Most pregnant women presented IgM that recognized more than one peptide, indicating multiplicity of P. vivax infection with co-infecting parasite clones per woman. Regarding recurrences, several women in the PI + 1R and PI ≥2R groups exhibited IgM responses against peptides of the same block 2 pvmsp1 haplotypes in successive episodes [Figure 2b and c].
The IgG reactivity was very low. Regarding the IgM to IgG switch, 8 women in the ONLY_PI group exhibited the switch [Figure 2a], 2 in the PI + 1R group showed the switch during the first infection, and only 1 showed the switch during the second episode [Figure 2b]. In the PI ≥2R group, 4 women showed the switch during the first infection, 1 during the second episode, 1 during the third episode, and 1 during the fourth episode [Figure 2c].
Regarding the number of peptides recognized by samples from the three groups of pregnant women, no differences were observed during the PI [Supplementary Figure 2 (1.6MB, tif) ]. However, the paired t-test indicated a reduction in the number of peptides recognized by samples from the PI + 1R group between the PI and the second malaria episode. For pregnant women in the PI ≥2R group, the repeated measures one-way ANOVA test revealed a significant reduction in the number of peptides recognized by IgM between the PI and the second and third malaria episodes (P = 0.012). No differences were observed for IgG between the second and third episodes.
IgM antipeptides as a tool for monitoring re-exposure to Plasmodium vivax-merozoite surface protein-1 block 2 haplotypes
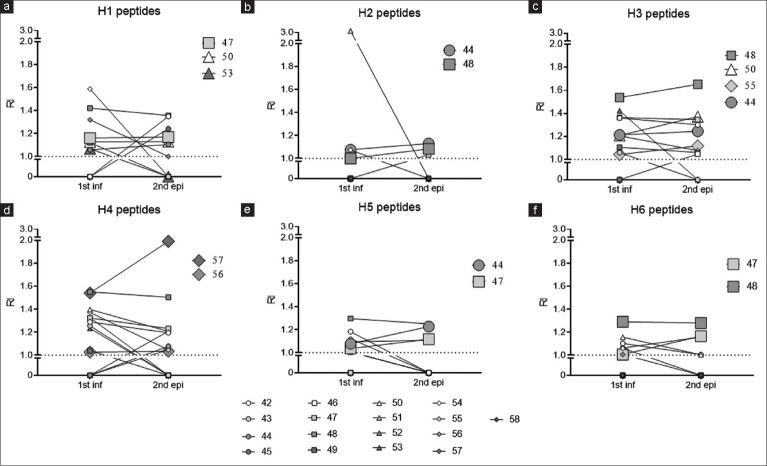
This assumption is better verified when IgM antibody levels against sets of peptides for each haplotype are followed up throughout the recurrences in the PI + 1R group [Figure 3] and in the PI ≥2R group [Figure 4]. Figure 3 shows the maintenance of IgM levels or even an increase in the second episode compared to the initial infection. Subject S-47 experienced her second episode 5 weeks after the PI, subject S-50’s second episode occurred 21 weeks later, and subject S-53 had her second episode 11 weeks after. All of them maintained or increased IgM levels against peptides of H1 in the second episode [Figure 3a]. Subject S-44 had her second episode 15 weeks after the PI, and subject S-48 had hers 6 weeks later, both showing increased IgM levels against peptides of H2 [Figure 3b]. Subject S-55 experienced her second episode 6 weeks later and exhibited increased IgM against peptides of H3, similar to subjects S-44, S-48, and S-50 [Figure 3c]. The same trend was observed with subjects S-56 and S-57 in relation to peptides of H4, with the period between the PI and the second episode being 18 and 14 weeks, respectively [Figure 3d]. Subjects S-44, S-47, and S-48 also had increases in IgM against peptides of H5 and H6 [Figure 3e and f].
Figure 3.
Antibody response to Plasmodium vivax-merozoite surface protein-11 (MSP1) block 2 specific haplotype peptides among pregnant women who have had a primary infection and a second malaria episode. Of the women, 17 had an initial infection and a relapse, which were confirmed by microscopy of the thick and thin smears of peripheral blood and polymerase chain reaction. Serology for IgM was performed against 6 haplotype-specific peptides of Plasmodium vivax-MSP1 Block 2 (a) H1 (b) H2 (c) H3 (d) H4 (e) H5 and (f) H6. Each woman was represented by a symbol and to the right of each graph are women who maintained or increased IgM levels on relapse relative to the initial infection
Figure 4.
Antibody response to Plasmodium vivax-merozoite surface protein-1 (MSP1) block 2 specific haplotype peptides among pregnant women who have had a primary infection and more than two malaria episodes. Of the women, 14 had two further episodes of malaria during pregnancy and these were confirmed by microscopy on thick and thin smears of peripheral blood and polymerase chain reaction. Serology for IgM was performed against 6 haplotype-specific peptides of Plasmodium vivax-MSP1 block 2 (a) P1 (b) P2 (c) P3 (d) P4 (e) P5 and (f) P6. Each woman was represented by a symbol and to the right of each graph are women who maintained or increased IgM levels on relapse relative to the initial infection
The increase in certain IgM antipeptides throughout the recurrences was most pronounced with women that more recurrence episodes (PI+>2R group). Figure 4 shows a pattern of maintenance or increase in subsequent episodes was observed with other peptides as well. Subjects S-61, S-64, and S-69 exhibited sustained IgM levels against peptides of H1 between the second and third episodes [Figure 4a]. Subjects S-60, S-62, and S-69 showed this trend with peptides of H2, especially subject S-60 for four episodes [Figure 4b]. Subjects S-59, S-61, S-62, S-64, S-69, and S-71 exhibited it with peptides of H3 [Figure 4c]. Similarly, subjects S-62, S-68, and S-69 displayed this pattern with peptides of H4 [Figure 4d], and S-61, S-62, S-65, and S-69 did so with peptides of H5 [Figure 4e]. Notably, subjects S-64, S-65, and S-69 exhibited this pattern with peptides of H6 [Figure 4f]. The sustained or increasing IgM levels throughout pregnancy likely indicate repeated exposures to the same populations of Block 2 pvmsp1 haplotypes.
DISCUSSION
Conventional genotyping methods often under- or overestimate the MOI and great progress has been made with next-generation sequencing.[1,14,15] However, since these targets are partial genomic regions, they may not fully represent the complete polymorphism in mixed infections.[14] That’s why the use of serological markers is also necessary to understand the exposition to clones of parasites. Here, we have developed a strategy that combines haplotype genotyping and antibody responses, both targeting Block 2 pvmsp1 haplotypes. Among the eight patients, IgM levels were consistent with the parasitic load corresponding to the “reads” of ADS. The combination of haplotype genotyping and antibody response to specific haplotype allows understand the population structure of P. vivax.
Serological markers for detecting recent exposure to P. vivax infection have been the subject of several studies.[16,17,18] Those based on robust IgG antibody responses do not distinguish clonal parasite populations.[17,19] Here, all samples infected by only one haplotype showed IgM antibody responses recognizing peptides from the same haplotype. Among samples infected by three haplotypes, the IgM levels were statistically higher against the predominant haplotypes. Conversely, IgG levels were not correlated with the parasite load estimated by the number of reads may be due to poorly immunogenic or elicit short-lived antibody responses.[20] IgM antibody response to sets of peptides of block 2 pvmsp1 haplotypes may be considered a good serological marker of exposure; however, more studies are needed.
Since hypnozontocidal treatment is contraindicated in pregnancy, pregnant women generally experience two or more parasitemia episodes.[21,22] We observed the persistence of IgM against the same sets of peptides in many pregnant women, as highlighted in Figures 3 and 4 with larger symbols. Note the IgM levels observed by two women, S-62 and S-70, against sets of peptides H3 and H4, respectively, well-characterize a re-exposure [Figure 4]. Despite IgM usually being considered short-lived according to studies,[17,18] its persistence in this study is important for detecting recent exposure to P. vivax, especially in shorter time frames. Another important finding was the low frequency of switching from IgM to IgG over successive episodes of recurrence [Figure 4b and c], which may be due to the poor immunogenicity of variant antigens,[20] in addition to the immunosuppression during pregnancy. Thus, IgM serology with our sets of peptides generated from the main haplotypes of PvMSP1 block 2 may be utilized as a serological marker for parasite clones and an auxiliary tool for determining relapse/reinfection epidemiology in vivax malaria.
The study had some limitations, including the lack of a genotyping method to confirm the relapses and the low reactivity of IgG in pregnant women against peptides in the ELISA when using a haplotype-specific peptide.
CONCLUSION
Our analysis suggests that IgM serology based on block 2 pvmsp1 haplotypes may be a useful system for observing the response against a specific haplotype, which may be used as a strategy for malaria control and elimination.
Research quality and ethics statement
This study received approval from the Institutional Review Board/Ethics Committee (Fundação de Medicina Tropical Dr. Heitor Vieira Dourado IRB# 42021515.0.3001.0005 and University of São Paulo IRB# 50158321.0.0000.5467).
Conflicts of interest
There are no conflicts of interest.
Exploration of Plasmodium vivax-merozoite surface protein-1 block 2 genetic diversity (a) A mosaic assembly of several block 2 sequences obtained from the GenBank database containing hybrid (or semi-conserved series) flanking random repeats. The mosaic was drawn using different colors for the conserved, hybrid, and repeat sequences to facilitate comprehension of diversity. Six allelic families sharing hybrid sequences up and downstream of random repeats
Comparison of the number of haplotype-specific peptides recognized. A number of peptides recognized through IgM (a) and IgG (b) in 41 women in the ONLY_primary infection (PI) group (who only had a malaria episode during pregnancy), 17 in the 1-PI + 1R group (had an initial infection and a relapse) and 14 in the PI ≥2R group (had an initial infection and two more relapse episodes). The Friedman test was used to compare the number of haplotype-specific peptides recognized between primary infection and second episode of pregnant women of PI + 1R and PI ≥2R group. nsNonsignificant
Funding Statement
Nil.
REFERENCES
- 1.Lin JT, Hathaway NJ, Saunders DL, Lon C, Balasubramanian S, Kharabora O, et al. Using amplicon deep sequencing to detect genetic signatures of Plasmodium vivax relapse. J Infect Dis. 2015;212:999–1008. doi: 10.1093/infdis/jiv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pacheco MA, Lopez-Perez M, Vallejo AF, Herrera S, Arévalo-Herrera M, Escalante AA. Multiplicity of infection and disease severity in Plasmodium vivax. PLoS Negl Trop Dis. 2016;10:e0004355. doi: 10.1371/journal.pntd.0004355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koepfli C, Ross A, Kiniboro B, Smith TA, Zimmerman PA, Siba P, et al. Multiplicity and diversity of Plasmodium vivax infections in a highly endemic region in Papua New Guinea. PLoS Negl Trop Dis. 2011;5:1–7. doi: 10.1371/journal.pntd.0001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira MU, Liu Q, Kaneko O, Kimura M, Tanabe K, Kimura EA, et al. Allelic diversity at the merozoite surface protein-1 locus of Plasmodium falciparum in clinical isolates from the Southwestern Brazilian Amazon. Am J Trop Med Hyg. 1998;59:474–80. doi: 10.4269/ajtmh.1998.59.474. [DOI] [PubMed] [Google Scholar]
- 5.Bharti PK, Shukla MM, Sharma YD, Singh N. Genetic diversity in the block 2 region of the merozoite surface protein-1 of Plasmodium falciparum in central India. Malar J. 2012;11:78. doi: 10.1186/1475-2875-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takala SL, Coulibaly D, Thera MA, Batchelor AH, Cummings MP, Escalante AA, et al. Extreme polymorphism in a vaccine antigen and risk of clinical malaria: Implications for vaccine development. Sci Transl Med. 2009;1:2ra5. doi: 10.1126/scitranslmed.3000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beeson JG, Drew DR, Boyle MJ, Feng G, Fowkes FJ, Richards JS. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev. 2016;40:343–72. doi: 10.1093/femsre/fuw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soares LA, Evangelista J, Orlandi PP, Almeida ME, de Sousa LP, Chaves Y, et al. Genetic diversity of MSP1 block 2 of Plasmodium vivax isolates from Manaus (central Brazilian Amazon) J Immunol Res. 2014;2014:671050. doi: 10.1155/2014/671050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Versiani FG, Almeida ME, Melo GC, Versiani FO, Orlandi PP, Mariúba LA, et al. High levels of IgG3 anti ICB2-5 in Plasmodium vivax-infected individuals who did not develop symptoms. Malar J. 2013;12:294. doi: 10.1186/1475-2875-12-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ataíde R, Murillo O, Dombrowski JG, Souza RM, Lima FA, Lima GF, et al. Malaria in pregnancy interacts with and alters the angiogenic profiles of the placenta. PLoS Negl Trop Dis. 2015;9:e0003824. doi: 10.1371/journal.pntd.0003824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dombrowski JG, Barateiro A, Peixoto EP, Barros AB, Souza RM, Clark TG, et al. Adverse pregnancy outcomes are associated with Plasmodium vivax malaria in a prospective cohort of women from the Brazilian Amazon. PLoS Negl Trop Dis. 2021;15:e0009390. doi: 10.1371/journal.pntd.0009390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dombrowski JG, Souza RM, Lima FA, Bandeira CL, Murillo O, Costa DS, et al. Association of malaria infection during pregnancy with head circumference of newborns in the Brazilian Amazon. JAMA Netw Open. 2019;2:e193300. doi: 10.1001/jamanetworkopen.2019.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brazil, Ministry of Health. Secretariat of Health Surveillance. Department of Immunization and Communicable Diseases. Practical Guide for Malaria Treatment in Brazil, 2nd edition, Brasília, Ministry of Health, 2021. (In Portuguese). https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/svsa/malaria/tratamento/guia_tratamento_malaria_2nov21_isbn_site.pdf/view. [Last accessed on 2024 Feb 07]
- 14.Zhong D, Koepfli C, Cui L, Yan G. Molecular approaches to determine the multiplicity of Plasmodium infections. Malar J. 2018;17:172. doi: 10.1186/s12936-018-2322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck HP, Wampfler R, Carter N, Koh G, Osorio L, Rueangweerayut R, et al. Estimation of the antirelapse efficacy of tafenoquine, Using Plasmodium vivax genotyping. J Infect Dis. 2016;213:794–9. doi: 10.1093/infdis/jiv508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutts JC, Powell R, Agius PA, Beeson JG, Simpson JA, Fowkes FJ. Immunological markers of Plasmodium vivax exposure and immunity: A systematic review and meta-analysis. BMC Med. 2014;12:150. doi: 10.1186/s12916-014-0150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longley RJ, White MT, Takashima E, Brewster J, Morita M, Harbers M, et al. Development and validation of serological markers for detecting recent Plasmodium vivax infection. Nat Med. 2020;26:741–9. doi: 10.1038/s41591-020-0841-4. [DOI] [PubMed] [Google Scholar]
- 18.Longley RJ, White MT, Brewster J, Liu ZS, Bourke C, Takashima E, et al. IgG antibody responses are preferential compared with IgM for use as serological markers for detecting recent exposure to Plasmodium vivax infection. Open Forum Infect Dis. 2021;8:ofab228. doi: 10.1093/ofid/ofab228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nogueira PA, Alves FP, Fernandez-Becerra C, Pein O, Santos NR, Pereira da Silva LH, et al. A reduced risk of infection with Plasmodium vivax and clinical protection against malaria are associated with antibodies against the N terminus but not the C terminus of merozoite surface protein 1. Infect Immun. 2006;74:2726–33. doi: 10.1128/IAI.74.5.2726-2733.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastos MS, da Silva-Nunes M, Malafronte RS, Hoffmann EH, Wunderlich G, Moraes SL, et al. Antigenic polymorphism and naturally acquired antibodies to Plasmodium vivax merozoite surface protein 1 in rural Amazonians. Clin Vaccine Immunol. 2007;14:1249–59. doi: 10.1128/CVI.00243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thanapongpichat S, McGready R, Luxemburger C, Day NP, White NJ, Nosten F, et al. Microsatellite genotyping of Plasmodium vivax infections and their relapses in pregnant and non-pregnant patients on the Thai-Myanmar border. Malar J. 2013;12:275. doi: 10.1186/1475-2875-12-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Perez M, Pacheco MA, Buriticá L, Escalante AA, Herrera S, Arévalo-Herrera M. Malaria in pregnancy: A passive surveillance study of pregnant women in low transmission areas of Colombia, Latin America. Malar J. 2016;15:66. doi: 10.1186/s12936-016-1125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exploration of Plasmodium vivax-merozoite surface protein-1 block 2 genetic diversity (a) A mosaic assembly of several block 2 sequences obtained from the GenBank database containing hybrid (or semi-conserved series) flanking random repeats. The mosaic was drawn using different colors for the conserved, hybrid, and repeat sequences to facilitate comprehension of diversity. Six allelic families sharing hybrid sequences up and downstream of random repeats
Comparison of the number of haplotype-specific peptides recognized. A number of peptides recognized through IgM (a) and IgG (b) in 41 women in the ONLY_primary infection (PI) group (who only had a malaria episode during pregnancy), 17 in the 1-PI + 1R group (had an initial infection and a relapse) and 14 in the PI ≥2R group (had an initial infection and two more relapse episodes). The Friedman test was used to compare the number of haplotype-specific peptides recognized between primary infection and second episode of pregnant women of PI + 1R and PI ≥2R group. nsNonsignificant