Abstract
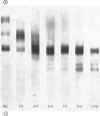
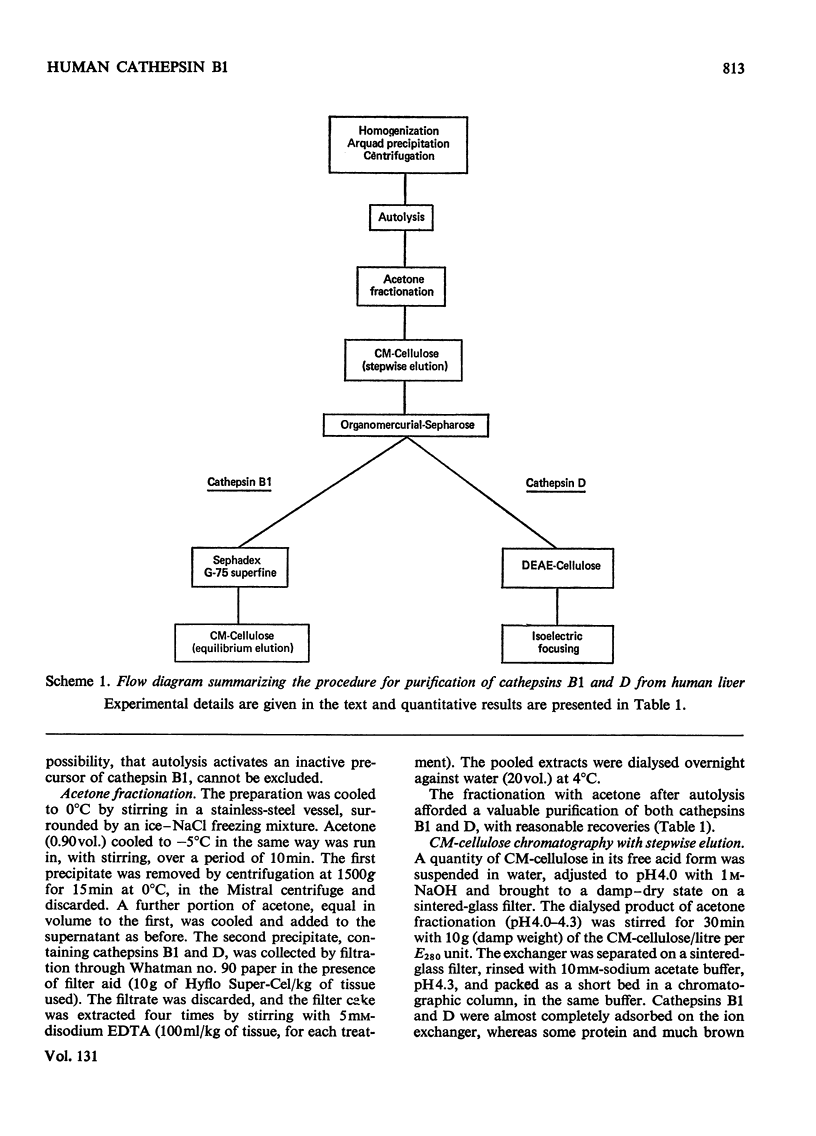
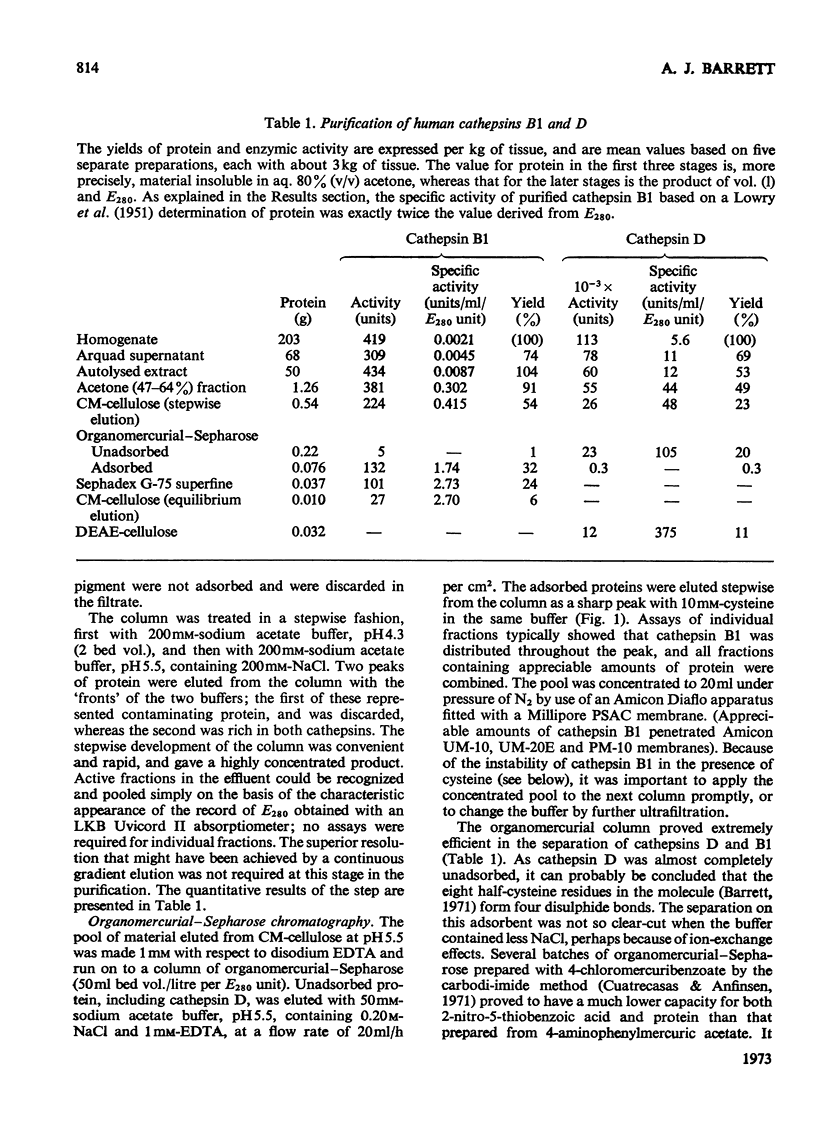
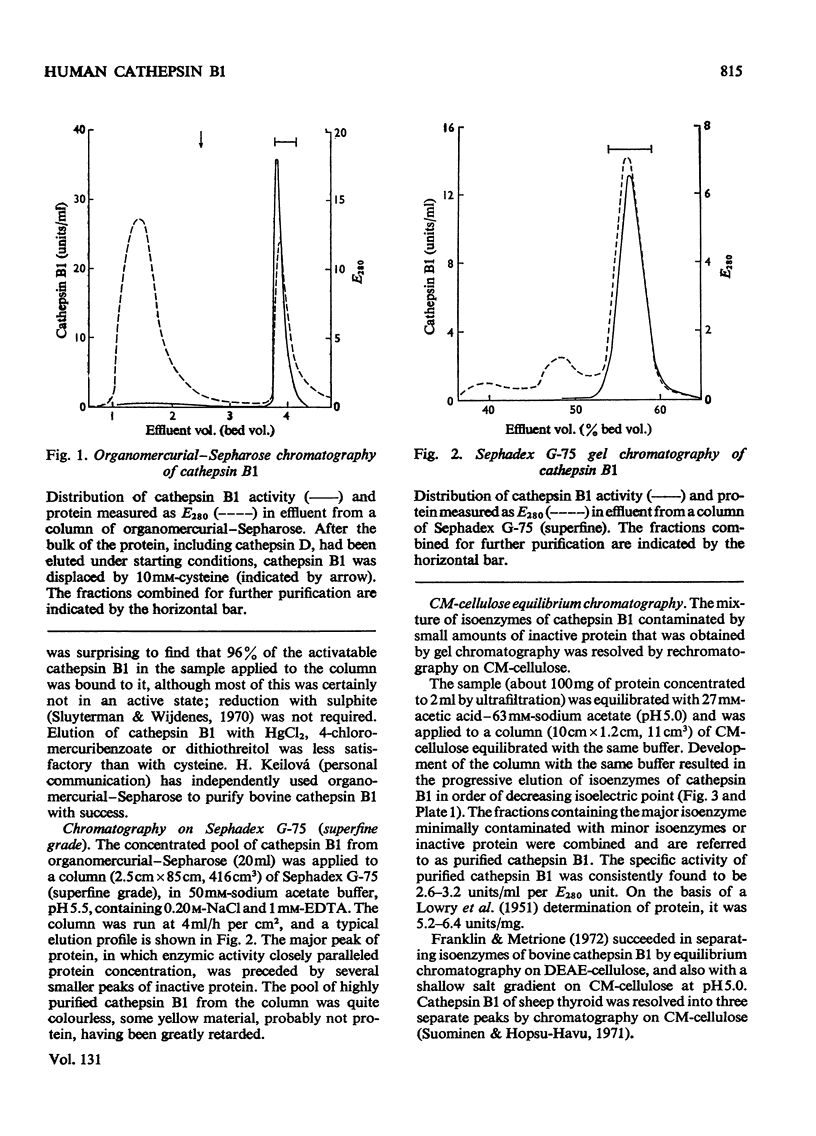
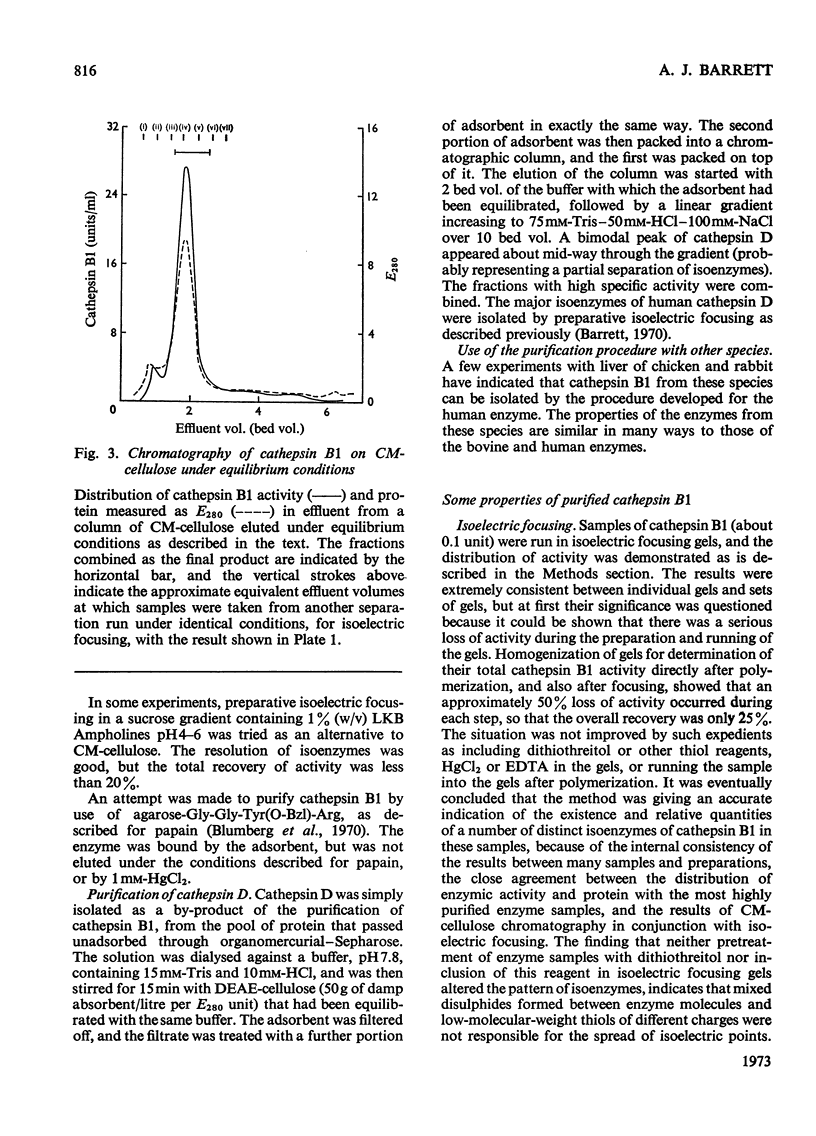
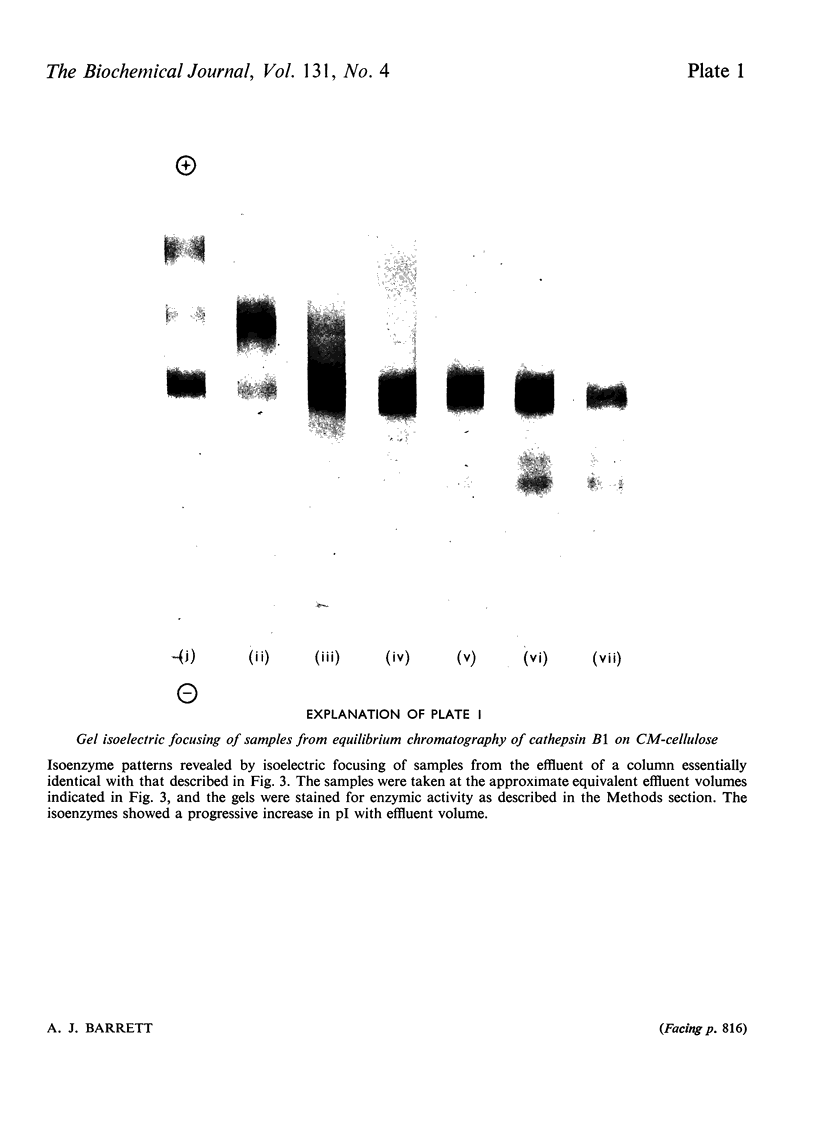
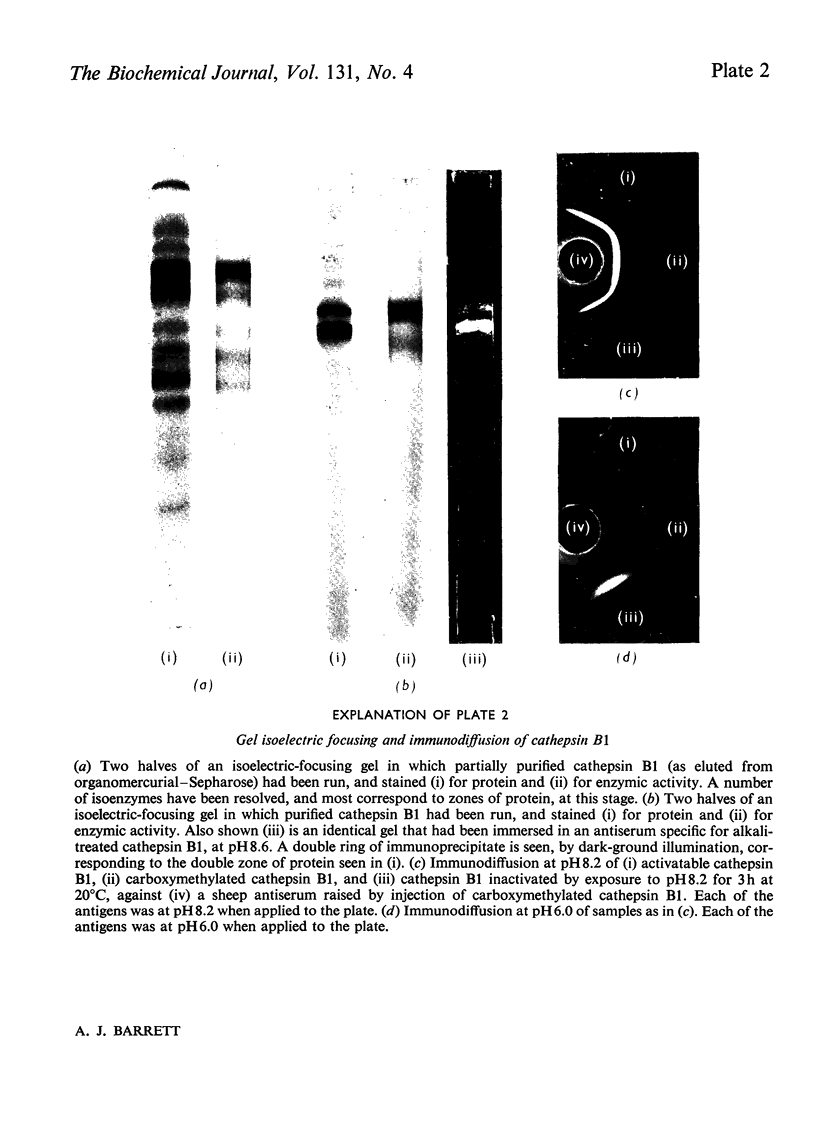
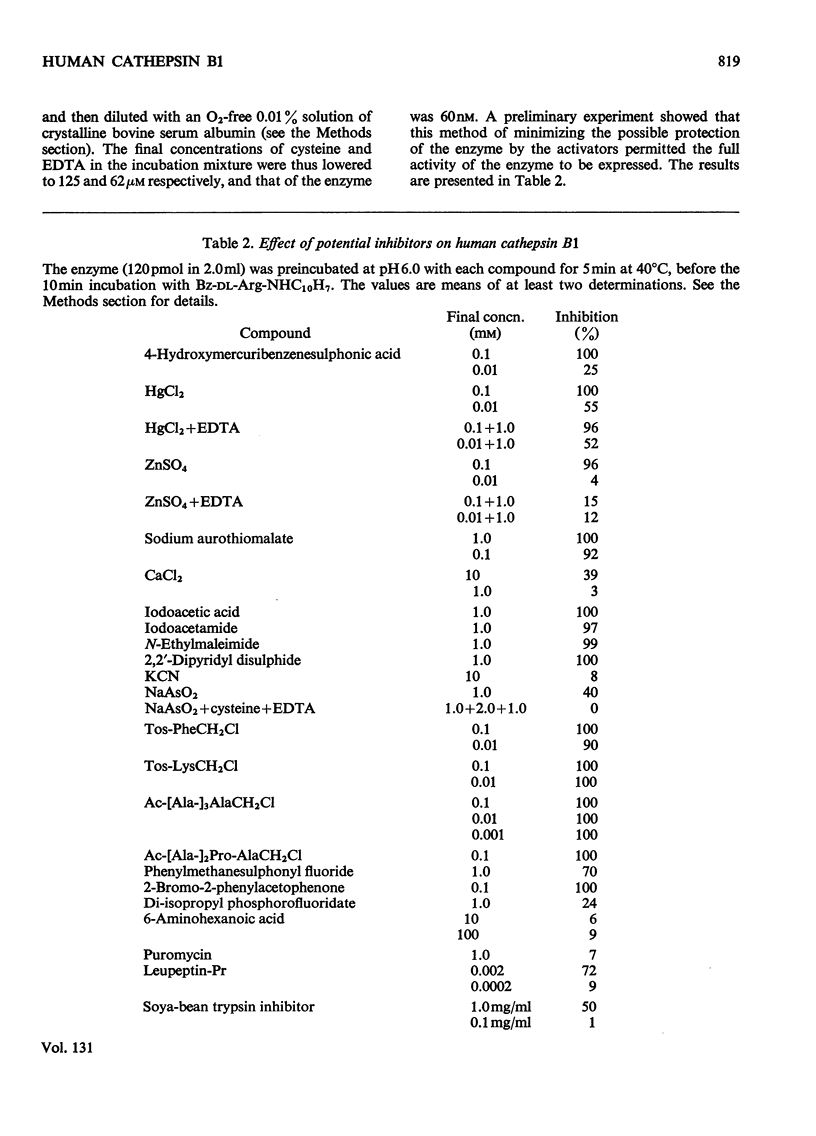
1. Cathepsin B1 was purified from human liver by a method involving autolysis, fractional precipitation with acetone, adsorption on, and stepwise elution from, CM-cellulose and an organomercurial adsorbent, gel chromatography and finally equilibrium chromatography on CM-cellulose. 2. The early stages of the procedure, including the use of the organomercurial adsorbent, were suitable for the simultaneous isolation of cathepsin D. The two cathepsins were sharply separated on the organomercurial column, and particular attention was given to the method for the preparation and use of this adsorbent. 3. A method is described for the staining of analytical isoelectric-focusing gels for cathepsin B1 activity, as well as protein. By this method it was shown that cathepsin B1 was represented by at least six isoenzymes during the greater part of the purification procedure. After the gel-chromatography step this group of isoenzymes was obtained essentially free of other proteins, in good yield. The isoenzymes were resolved from this mixture by chromatography on CM-cellulose. The purified enzyme was stable for several weeks at slightly acid pH values in the absence of thiol compounds; it was unstable above pH7. 4. The pI values of the isoenzymes of cathepsin B1 extended from pH4.5 to 5.5, that of the major isoenzyme tending to increase from 5.0 to 5.2 during the purification procedure. Gel chromatography indicated a molecular weight of 27500 for all of the isoenzymes, whereas polyacrylamide-gel electrophoresis in the presence of sodium dodecyl sulphate gave a value of 24000. 5. An antiserum raised in sheep against the purified enzyme reacted specifically with the alkali-denatured molecule. Purified cathepsin B1 contained no material precipitable by an anti-(human cathepsin D) serum. 6. The enzyme hydrolysed several N-substituted derivatives of l-arginine 2-naphthylamide, as well as haemoglobin, azo-haemoglobin, azo-globin and azo-casein. Greatest activity was obtained near pH6.0. 7. The sensitivity of human cathepsin B1 to chemical inhibitors was generally similar to that of other thiol proteinases. The enzyme was inactivated by the chloromethyl ketones derived from tosylphenylalanine, tosyl-lysine, acetyltetra-alanine and acetyldialanylprolylalanine. 8. The hydrolysis of α-N-benzoyl-dl-arginine 2-naphthylamide by extracts of human liver at pH6 was attributable entirely to cathepsin B1.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y. The degradation of cartilage matrix by an intracellular protease. Biochem J. 1964 Dec;93(3):611–618. doi: 10.1042/bj0930611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon R. The reaction of papain with antipapain. Immunochemistry. 1965 Jun;2(2):107–114. doi: 10.1016/0019-2791(65)90012-1. [DOI] [PubMed] [Google Scholar]
- Axén R., Ernback S. Chemical fixation of enzymes to cyanogen halide activated polysaccharide carriers. Eur J Biochem. 1971 Feb 1;18(3):351–360. doi: 10.1111/j.1432-1033.1971.tb01250.x. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. A new assay for cathepsin B1 and other thiol proteinases. Anal Biochem. 1972 May;47(1):280–293. doi: 10.1016/0003-2697(72)90302-8. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Dingle J. T. The inhibition of tissue acid proteinases by pepstatin. Biochem J. 1972 Apr;127(2):439–441. doi: 10.1042/bj1270439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Poole A. R. Unsuitability of leucine naphthylamide for the histochemical demonstration of lysosomal proteolytic activity. Nature. 1969 Oct 18;224(5216):279–280. doi: 10.1038/224279a0. [DOI] [PubMed] [Google Scholar]
- Blumberg S., Schechter I., Berger A. The purification of papain by affinity chromatography. Eur J Biochem. 1970 Jul;15(1):97–102. doi: 10.1111/j.1432-1033.1970.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. A novel reactivity of papain and a convenient active site titration in the presence of other thiols. FEBS Lett. 1970 Jul 29;9(2):113–116. doi: 10.1016/0014-5793(70)80327-1. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- De Lumen B. O., Tappel A. L. -N-benzoylarginine- -naphthylamide amidohydrolase of rat liver lysosomes. J Biol Chem. 1972 Jun 10;247(11):3552–3557. [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J., Weston P. D. Cathepsin D. Characteristics of immunoinhibition and the confirmation of a role in cartilage breakdown. Biochem J. 1971 Jun;123(1):1–13. doi: 10.1042/bj1230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelmaier P., Hübner H., Otto K. Cathepsins B1 and B2 in various organs of the rat. Enzymologia. 1972 May 31;42(5):363–375. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Franklin S. G., Metrione R. M. Chromatographic evidence for the existence of multiple forms of cathepsin B1. Biochem J. 1972 Mar;127(1):207–213. doi: 10.1042/bj1270207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOULD N. R., LIENER I. E. REACTION OF FICIN WITH DIISOPROPYLPHOSPHOROFLUORIDATE. EVIDENCE FOR A CONTAMINATING INHIBITOR. Biochemistry. 1965 Jan;4:90–98. doi: 10.1021/bi00877a016. [DOI] [PubMed] [Google Scholar]
- GREENBAUM L. M., FRUTON J. S. Purification and properties of beef spleen cathepsin B. J Biol Chem. 1957 May;226(1):173–180. [PubMed] [Google Scholar]
- Ikezawa H., Aoyagi T., Takeuchi T., Umezawa H. Effect of protease inhibitors of actinomycetes on lysosomal peptide-hydrolases from swine liver. J Antibiot (Tokyo) 1971 Jul;24(7):488–490. doi: 10.7164/antibiotics.24.488. [DOI] [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- Keilová H., Keil B. Isolation and specificity of cathepsin B. FEBS Lett. 1969 Aug;4(4):295–298. doi: 10.1016/0014-5793(69)80260-7. [DOI] [PubMed] [Google Scholar]
- Keilová H., Turková J. Analogy between active sites of cathepsin B(1) and papain. FEBS Lett. 1970 Dec 11;11(4):287–288. doi: 10.1016/0014-5793(70)80550-6. [DOI] [PubMed] [Google Scholar]
- Klein I. B., Kirsch J. F. The mechanism of the activation of papain. Biochem Biophys Res Commun. 1969 Mar 10;34(5):575–581. doi: 10.1016/0006-291x(69)90776-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McDonald J. K., Reilly T. J., Zeitman B. B., Ellis S. Dipeptidyl arylamidase II of the pituitary. Properties of lysylalanyl-beta-naphthylamide hydrolysis: inhibition by cations, distribution in tissues, and subcellular localization. J Biol Chem. 1968 Apr 25;243(8):2028–2037. [PubMed] [Google Scholar]
- McDonald J. K., Zeitman B. B., Ellis S. Leucine naphthylamide: an inappropriate [corrected] substrate for the histochemical detection of cathepsins B and B'. Nature. 1970 Mar 14;225(5237):1048–1049. doi: 10.1038/2251048a0. [DOI] [PubMed] [Google Scholar]
- Misaka E., Tappel A. L. Inhibition studies of cathepsins A, B, C and D from rat liver lysosomes. Comp Biochem Physiol B. 1971 Apr 15;38(4):651–662. doi: 10.1016/0305-0491(71)90268-9. [DOI] [PubMed] [Google Scholar]
- Otto K., Bhakdi S. Zur Kenntnis des Kathepsins B': Spezifität und Eigenschaften. Hoppe Seylers Z Physiol Chem. 1969 Dec;350(12):1577–1588. [PubMed] [Google Scholar]
- Otto K., Schepers P. Uber die katheptische Inaktivierung einiger Enzyme der Rattenleber, insbesondere der Glucokinase. Hoppe Seylers Z Physiol Chem. 1967 May;348(5):482–490. [PubMed] [Google Scholar]
- Otto K. Uber ein neues Kathepsin. Reinigung aus Rindermilz, Eigenschaften, sowie Vergleich mit Kathepsin B. Hoppe Seylers Z Physiol Chem. 1967 Nov;348(11):1449–1460. [PubMed] [Google Scholar]
- Powers J. C., Tuhy P. M. Active-site specific inhibitors of elastase. J Am Chem Soc. 1972 Sep 6;94(8):6544–6545. doi: 10.1021/ja00773a049. [DOI] [PubMed] [Google Scholar]
- Sakai T., Gross J. Some properties of the products of reaction of tadpole collagenase with collagen. Biochemistry. 1967 Feb;6(2):518–528. doi: 10.1021/bi00854a021. [DOI] [PubMed] [Google Scholar]
- Sluyterman L. A. The activation reaction of papain. Biochim Biophys Acta. 1967 Jul 11;139(2):430–438. doi: 10.1016/0005-2744(67)90046-0. [DOI] [PubMed] [Google Scholar]
- Sluyterman L. A., Wijdenes J. An agarose mercurial column for the separation of mercaptopapain and nonmercaptopapain. Biochim Biophys Acta. 1970 Mar 31;200(3):593–595. doi: 10.1016/0005-2795(70)90122-4. [DOI] [PubMed] [Google Scholar]
- Snellman O. Cathepsin B, the lysosomal thiol proteinase of calf liver. Biochem J. 1969 Oct;114(4):673–678. doi: 10.1042/bj1140673b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Inhibition by alpha-macroglobulin and other serum proteins. Biochem J. 1973 Apr;131(4):823–831. doi: 10.1042/bj1310823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suominen J., Hopsu-Havu V. K. Cathepsin B' in the thyroid gland. Acta Chem Scand. 1971;25(7):2531–2540. doi: 10.3891/acta.chem.scand.25-2531. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whitaker J. R., Perez-Villase ñor J. Chemical modification of papain. I. Reaction with the chloromethyl ketones of phenylalanine and lysine and with phenylmethyl-sulfonyl fluoride. Arch Biochem Biophys. 1968 Mar 20;124(1):70–78. doi: 10.1016/0003-9861(68)90304-4. [DOI] [PubMed] [Google Scholar]