Abstract
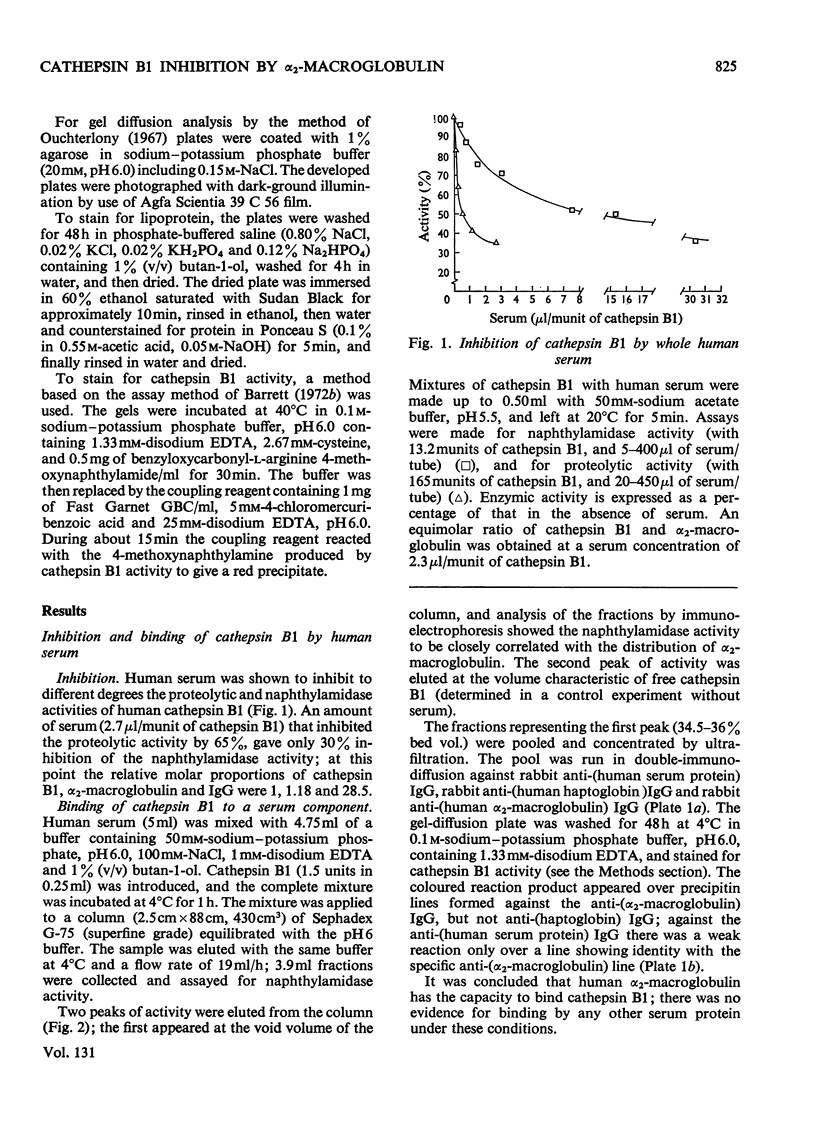
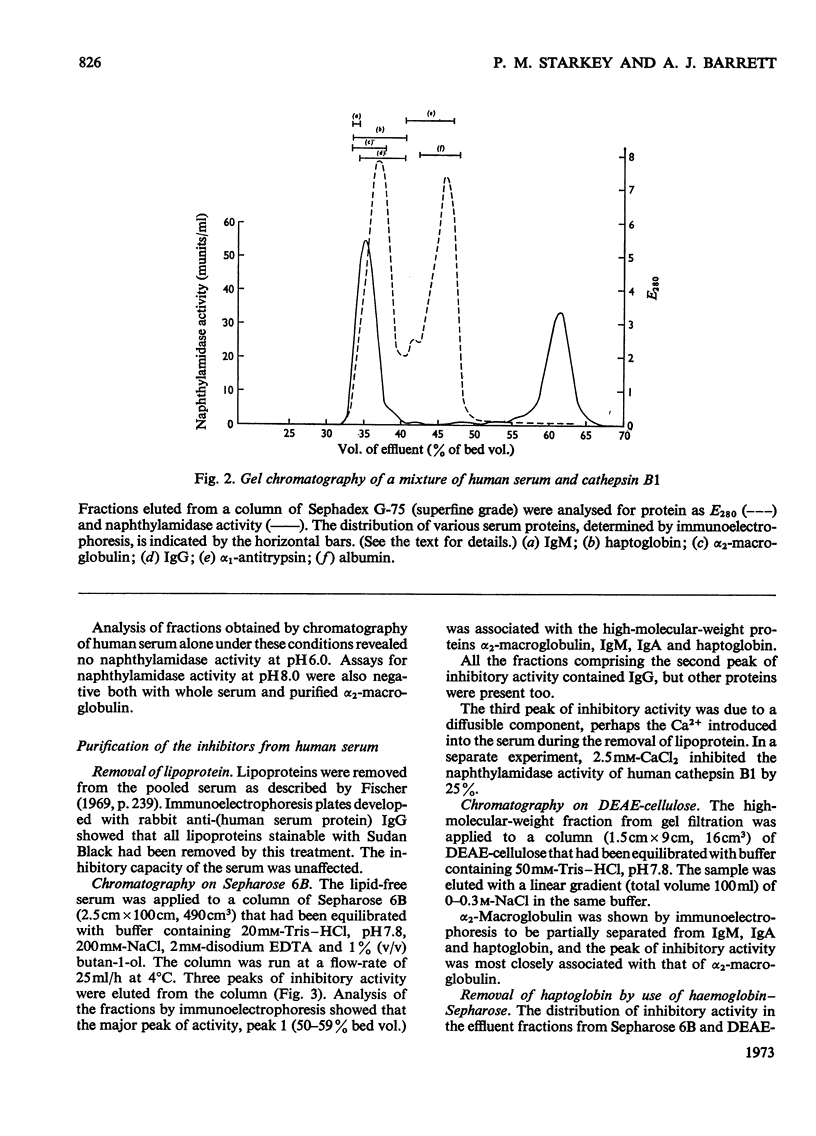
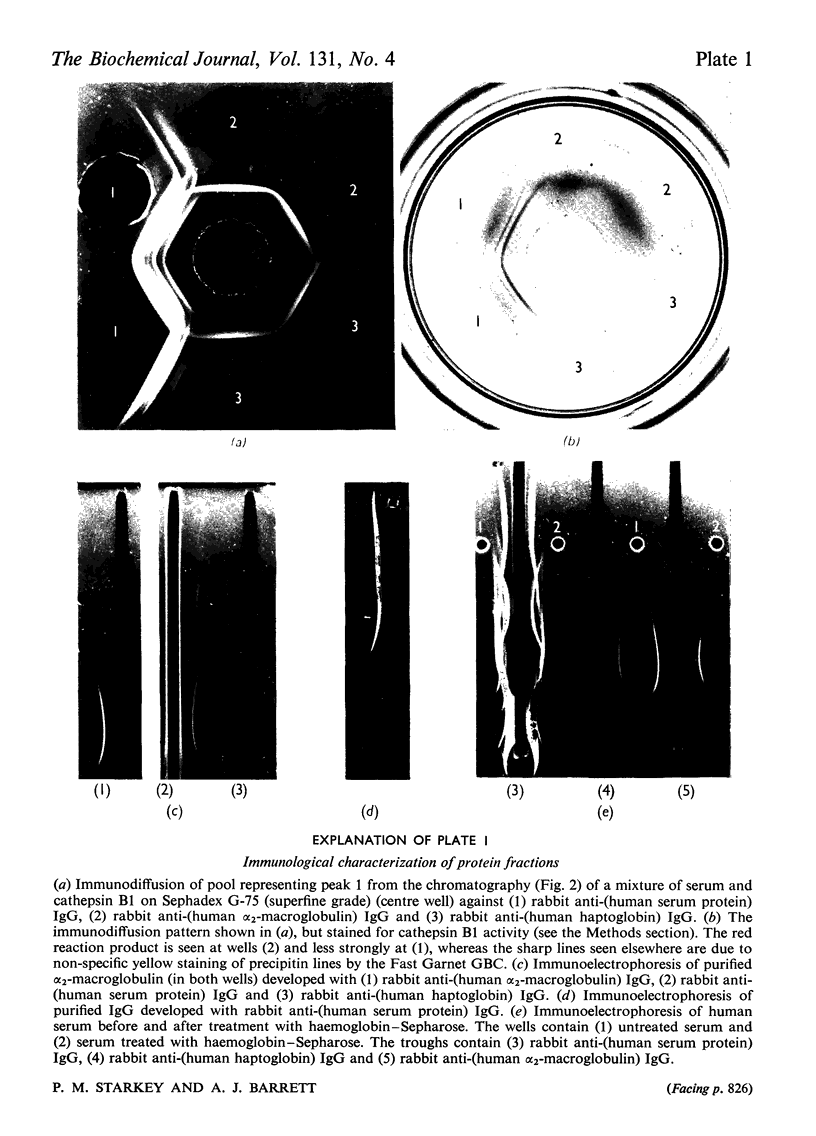
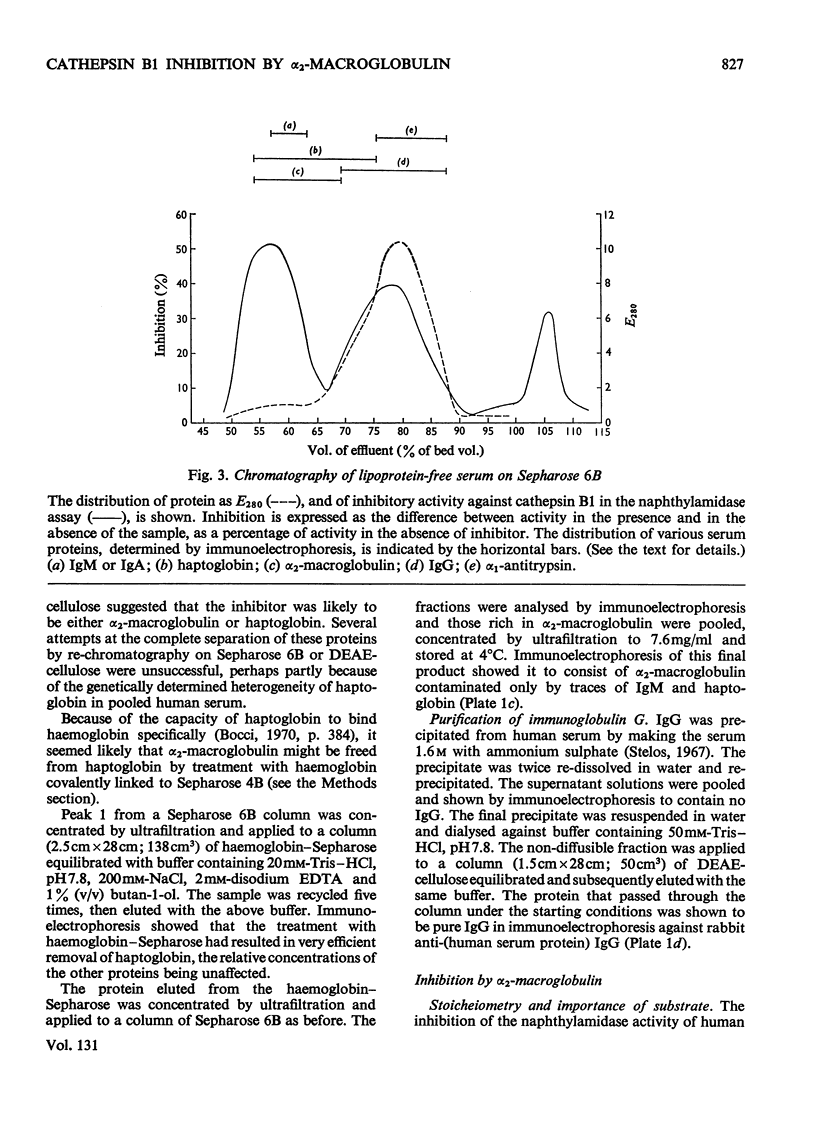
1. Normal human serum was found to inhibit human cathepsin B1. 2. The major inhibitor present in serum was purified and identified as α2-macroglobulin. 3. α2-Macroglobulin was found to bind cathepsin B1 in an approximately 1:1 molar ratio. When bound, the enzyme retained about 50% of its proteolytic activity, and up to 80% of its activity against α-N-benzoyl-dl-arginine 2-naphthylamide. 4. Pretreatment of α2-macroglobulin with cathepsin B1 inactivated by exposure to pH8.5 or iodoacetic acid, in large molar excess, did not prevent the subsequent binding of active enzyme. Active enzyme, once bound, was not protected from inhibition by 1-chloro-4-phenyl-3-tosylamido-l-butan-2-one. 5. Cathepsin B1 was also inhibited by human immunoglobulin G, at high concentration. 6. Because it had been suggested that haptoglobin is responsible for the inhibition of `cathepsin B' by serum, a method was devised for the selective removal of haptoglobin from mixtures of serum proteins by adsorption on haemoglobin covalently linked to Sepharose. No evidence was obtained that haptoglobin has any inhibitory activity against the enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Ernback S. Chemical fixation of enzymes to cyanogen halide activated polysaccharide carriers. Eur J Biochem. 1971 Feb 1;18(3):351–360. doi: 10.1111/j.1432-1033.1971.tb01250.x. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. A new assay for cathepsin B1 and other thiol proteinases. Anal Biochem. 1972 May;47(1):280–293. doi: 10.1016/0003-2697(72)90302-8. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumstark J. S. Studies on the elastase-serum protein interaction. II. On the digestion of human a2-macroglobulin, an elastase inhibitor, by elastase. Biochim Biophys Acta. 1970 May 26;207(2):318–330. doi: 10.1016/0005-2795(70)90024-3. [DOI] [PubMed] [Google Scholar]
- Brubacher L. J., Bender M. L. The preparation and properties of trans-cinnamoyl-papain. J Am Chem Soc. 1966 Dec 20;88(24):5871–5880. doi: 10.1021/ja00976a032. [DOI] [PubMed] [Google Scholar]
- Counitchansky Y., Berthillier G., Got R. Mise en évidence et caractérisation des complexes formés entre les immunoglobulines A (IgA) du colostrum humain et la trypsine ou la chymotrypsine. Clin Chim Acta. 1970 Oct;30(1):83–92. doi: 10.1016/0009-8981(70)90196-8. [DOI] [PubMed] [Google Scholar]
- Dolovich J., Wicher V. The binding of Bacillus subtilis alkaline proteinases to alpha-2-macroglobulin. J Lab Clin Med. 1971 Jun;77(6):951–957. [PubMed] [Google Scholar]
- Dunn J. T., Spiro R. G. The alpha 2-macroglobulin of human plasma. I. Isolation and composition. J Biol Chem. 1967 Dec 10;242(23):5549–5555. [PubMed] [Google Scholar]
- Frénoy J. P., Razafimahaleo E., Bourrillon R. Etudes sur la structure de L'a 2 -macroglobuline humaine. 3. Isolement et caractérisation d'une sous-unité. Biochim Biophys Acta. 1972 Jan 26;257(1):111–121. [PubMed] [Google Scholar]
- RATCLIFF A. P., HARDWICKE J. ESTIMATION OF SERUM HAEMOGLOBIN-BINDING CAPACITY (HAPTOGLOBIN) ON SEPHADEX G.100. J Clin Pathol. 1964 Nov;17:676–679. doi: 10.1136/jcp.17.6.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuch M. Les antiprotéases du plasma. Rev Fr Transfus. 1971 Mar;14(1):61–82. doi: 10.1016/s0035-2977(71)80006-8. [DOI] [PubMed] [Google Scholar]



