Abstract
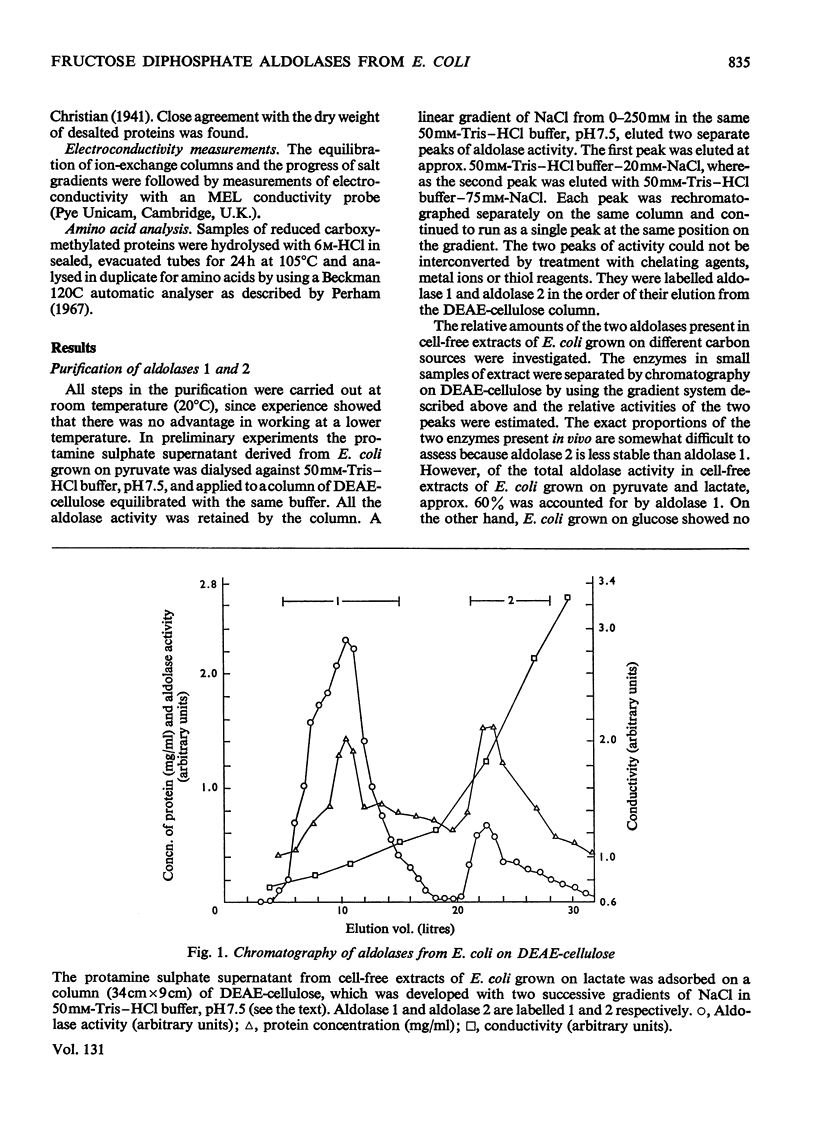
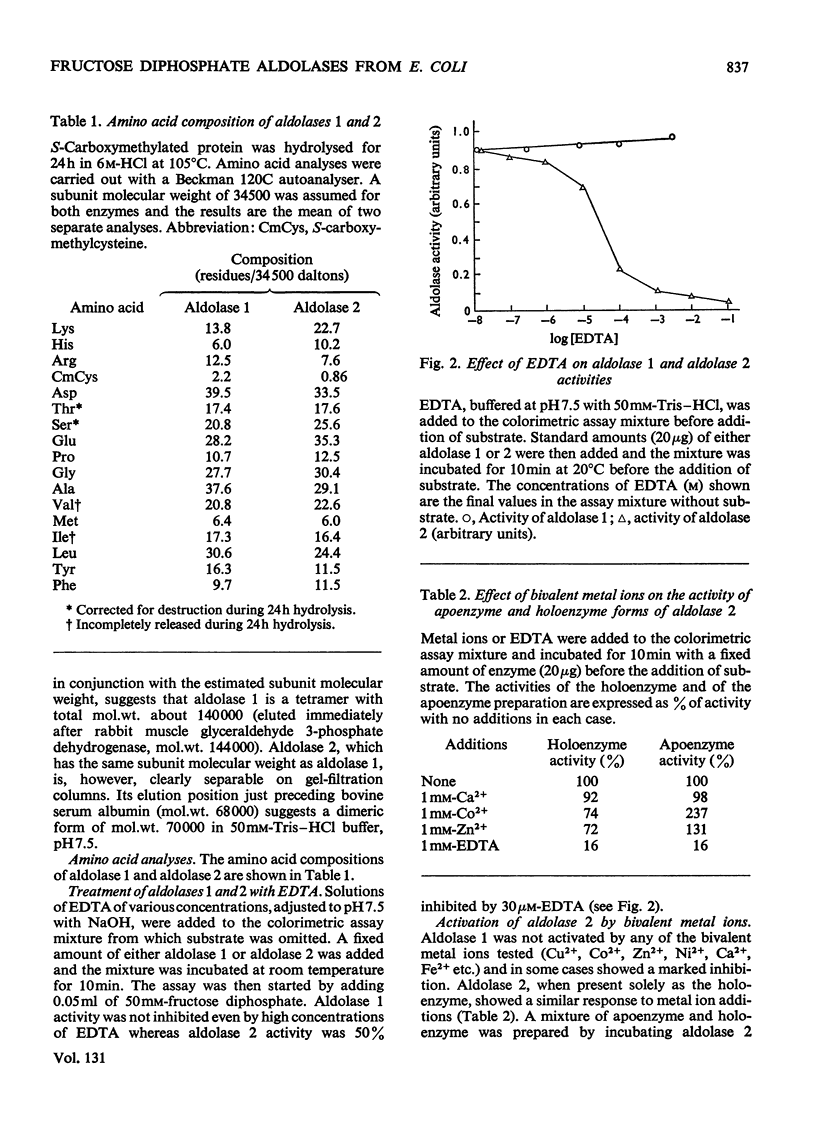
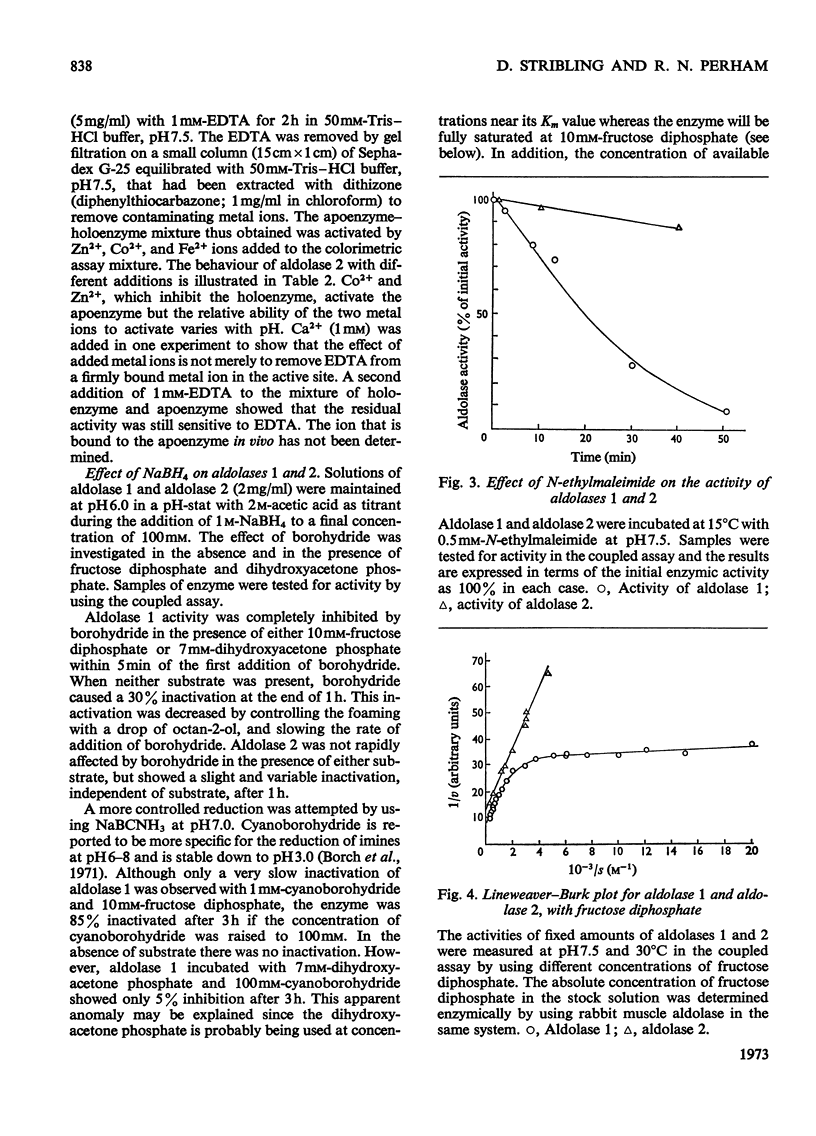
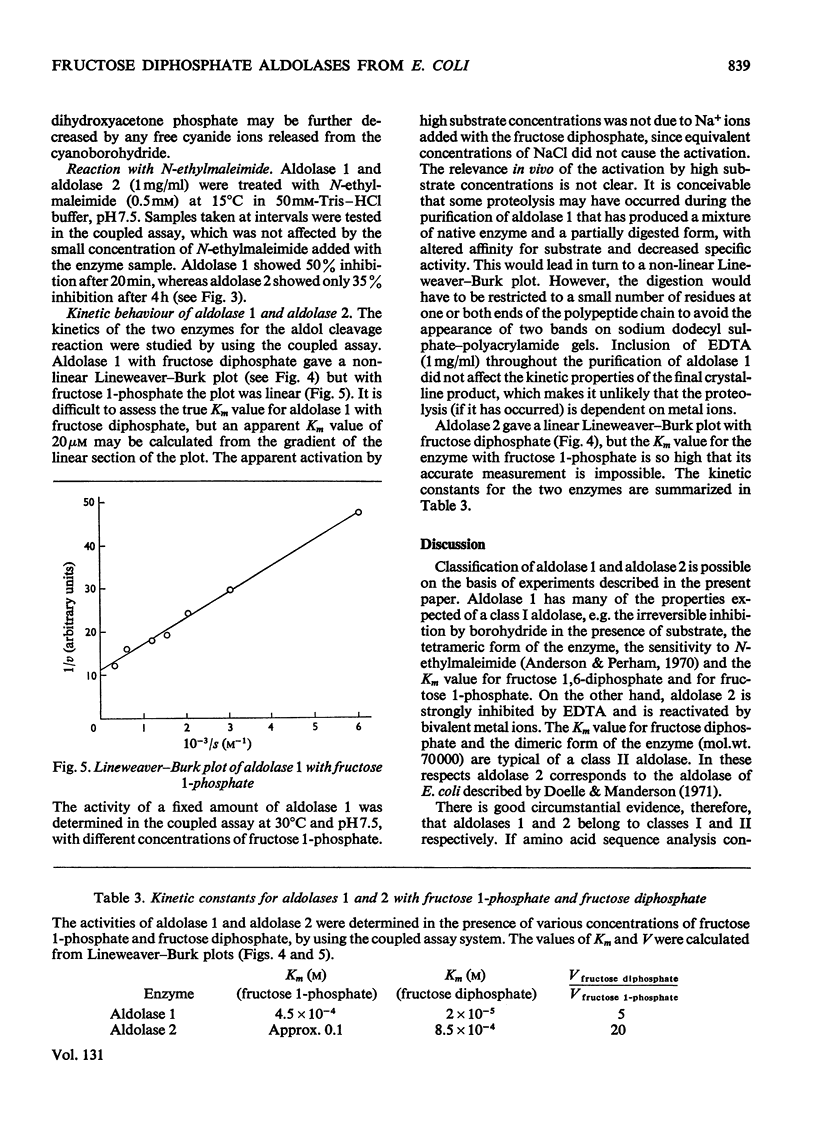
Two fructose diphosphate aldolases (EC 4.1.2.13) were detected in extracts of Escherichia coli (Crookes' strain) grown on pyruvate or lactate. The two enzymes can be resolved by chromatography on DEAE-cellulose at pH7.5, or by gel filtration on Sephadex G-200, and both have been obtained in a pure state. One is a typical bacterial aldolase (class II) in that it is strongly inhibited by metal-chelating agents and is reactivated by bivalent metal ions, e.g. Ca2+, Zn2+. It is a dimer with a molecular weight of approx. 70000, and the Km value for fructose diphosphate is about 0.85mm. The other aldolase is not dependent on metal ions for its activity, but is inhibited by reduction with NaBH4 in the presence of substrate. The Km value for fructose diphosphate is about 20μm (although the Lineweaver–Burk plot is not linear) and the enzyme is probably a tetramer with molecular weight approx. 140000. It has been crystallized. On the basis of these properties it is tentatively assigned to class I. The appearance of a class I aldolase in bacteria was unexpected, and its synthesis in E. coli is apparently favoured by conditions of gluconeogenesis. Only aldolase of class II was found in E. coli that had been grown on glucose. The significance of these results for the evolution of fructose diphosphate aldolases is briefly discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J., Perham R. N. The reactivity of thiol groups and the subunit structure of aldolase. Biochem J. 1970 Apr;117(2):291–298. doi: 10.1042/bj1170291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOSTEIN R., RUTTER W. J. COMPARATIVE STUDIES OF LIVER AND MUSCLE ALDOLASE. II. IMMUNOCHEMICAL AND CHROMATOGRAPHIC DIFFERENTIATION. J Biol Chem. 1963 Oct;238:3280–3285. [PubMed] [Google Scholar]
- DeVries G. H., Binkley S. B. N-acetylneuraminic acid aldolase of Clostridium perfringens: purification, properties and mechanism of action. Arch Biochem Biophys. 1972 Jul;151(1):234–242. doi: 10.1016/0003-9861(72)90493-6. [DOI] [PubMed] [Google Scholar]
- Doelle H. W., Manderson G. J. Comparative studies of fructose 1,6-diphosphate aldolase from Escherichia coli 518 and Lactobacillus casei var. rhamnosus ATCC 7469. Antonie Van Leeuwenhoek. 1971;37(1):21–31. doi: 10.1007/BF02218464. [DOI] [PubMed] [Google Scholar]
- Forcina B. G., Perham R. N. Amino acid sequence homology between muscle and liver aldolases. FEBS Lett. 1971 Oct 15;18(1):59–63. doi: 10.1016/0014-5793(71)80406-4. [DOI] [PubMed] [Google Scholar]
- GRAZI E., MELOCHE H., MARTINEZ G., WOOD W. A., HORECKER B. L. Evidence for Schiff base formation in enzymatic aldol condensations. Biochem Biophys Res Commun. 1963 Jan 18;10:4–10. doi: 10.1016/0006-291x(63)90257-2. [DOI] [PubMed] [Google Scholar]
- Gibbons I., Anderson P. J., Perham R. N. Amino acid sequence homology in the active site of rabbit and sturgeon muscle aldolases. FEBS Lett. 1970 Sep 18;10(1):49–53. doi: 10.1016/0014-5793(70)80413-6. [DOI] [PubMed] [Google Scholar]
- Gibbons I., Perham R. N. The reaction of aldolase with 2-methylmaleic anhydride. Biochem J. 1970 Mar;116(5):843–849. doi: 10.1042/bj1160843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini A. M., Cremona T., Preddie E. C. The aldolases of Chlamydomonas reinhardii. Arch Biochem Biophys. 1971 Sep;146(1):249–255. doi: 10.1016/s0003-9861(71)80062-0. [DOI] [PubMed] [Google Scholar]
- Guha A., Lai C. Y., Horecker B. L. Lobster muscle aldolase: isolation, properties and primary structure at the substrate-binding site. Arch Biochem Biophys. 1971 Dec;147(2):692–706. doi: 10.1016/0003-9861(71)90429-2. [DOI] [PubMed] [Google Scholar]
- HORECKER B. L., ROWLEY P. T., GRAZI E., CHENG T., TCHOLA O. THE MECHANISM OF ACTION OF ALDOLASES. IV. LYSINE AS THE SUBSTRATE-BINDING SITE. Biochem Z. 1963;338:36–51. [PubMed] [Google Scholar]
- Ingram J. M., Wood W. A. The mechanism of 2-keto-3-deoxy-6-phosphogluconic aldolase. II. Studies of the partial reactions with substrate analogues. J Biol Chem. 1966 Jul 25;241(14):3256–3261. [PubMed] [Google Scholar]
- Kobes R. D., Simpson R. T., Vallee R. L., Rutter W. J. A functional role of metal ions in a class II aldolase. Biochemistry. 1969 Feb;8(2):585–588. doi: 10.1021/bi00830a018. [DOI] [PubMed] [Google Scholar]
- Lebherz H. G. Stability of quaternary structure of mammalian and avian fructose diphosphate aldolases. Biochemistry. 1972 Jun 6;11(12):2243–2250. doi: 10.1021/bi00762a006. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Horecker B. L. The mechanism of action of aldolases. Adv Enzymol Relat Areas Mol Biol. 1968;31:125–181. doi: 10.1002/9780470122761.ch4. [DOI] [PubMed] [Google Scholar]
- Perham R. N. A diagonal paper-electrophoretic technique for studying amino acid sequences around the cysteine and cystine residues of proteins. Biochem J. 1967 Dec;105(3):1203–1207. doi: 10.1042/bj1051203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perham R. N., Thomas J. O. The subunit molecular weights of the alpha-ketoacid dehydrogenase multienzyme complexes from E. coli. FEBS Lett. 1971 Jun 2;15(1):8–12. doi: 10.1016/0014-5793(71)80066-2. [DOI] [PubMed] [Google Scholar]
- RUTTER W. J. EVOLUTION OF ALDOLASE. Fed Proc. 1964 Nov-Dec;23:1248–1257. [PubMed] [Google Scholar]
- Ribereau-Gayon G., Ramasarma T., Horecker B. L. Fructose diphosphate aldolase of spinach leaf: primary structure around the substrate-binding site. Arch Biochem Biophys. 1971 Nov;147(1):343–348. doi: 10.1016/0003-9861(71)90343-2. [DOI] [PubMed] [Google Scholar]
- Russell G. K., Gibbs M. Partial purification and characterization of two fructose diphosphate aldolases from Chlamydomonas mundana. Biochim Biophys Acta. 1967 Jan 11;132(1):145–154. doi: 10.1016/0005-2744(67)90200-8. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Maizel J. V., Jr Molecular weight estimation of polypeptides by SDS-polyacrylamide gel electrophoresis: further data concerning resolving power and general considerations. Anal Biochem. 1969 Jun;29(3):505–514. doi: 10.1016/0003-2697(69)90335-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


