Abstract
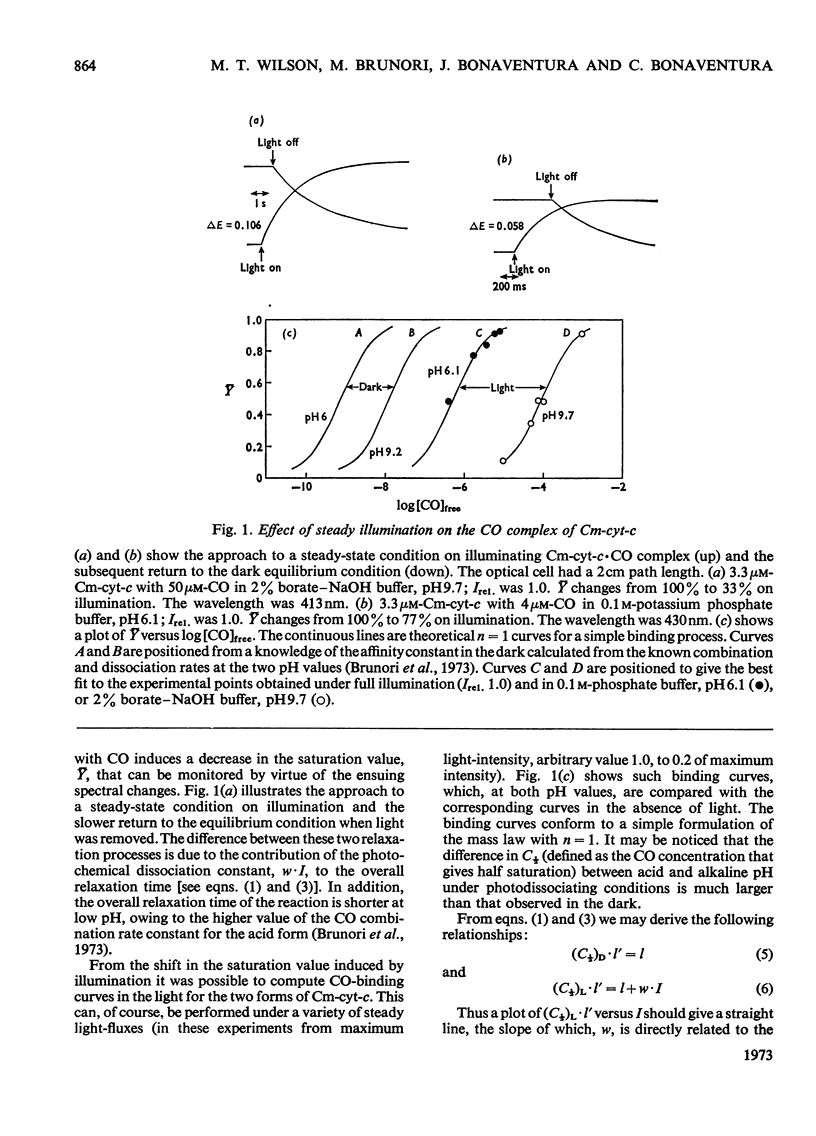
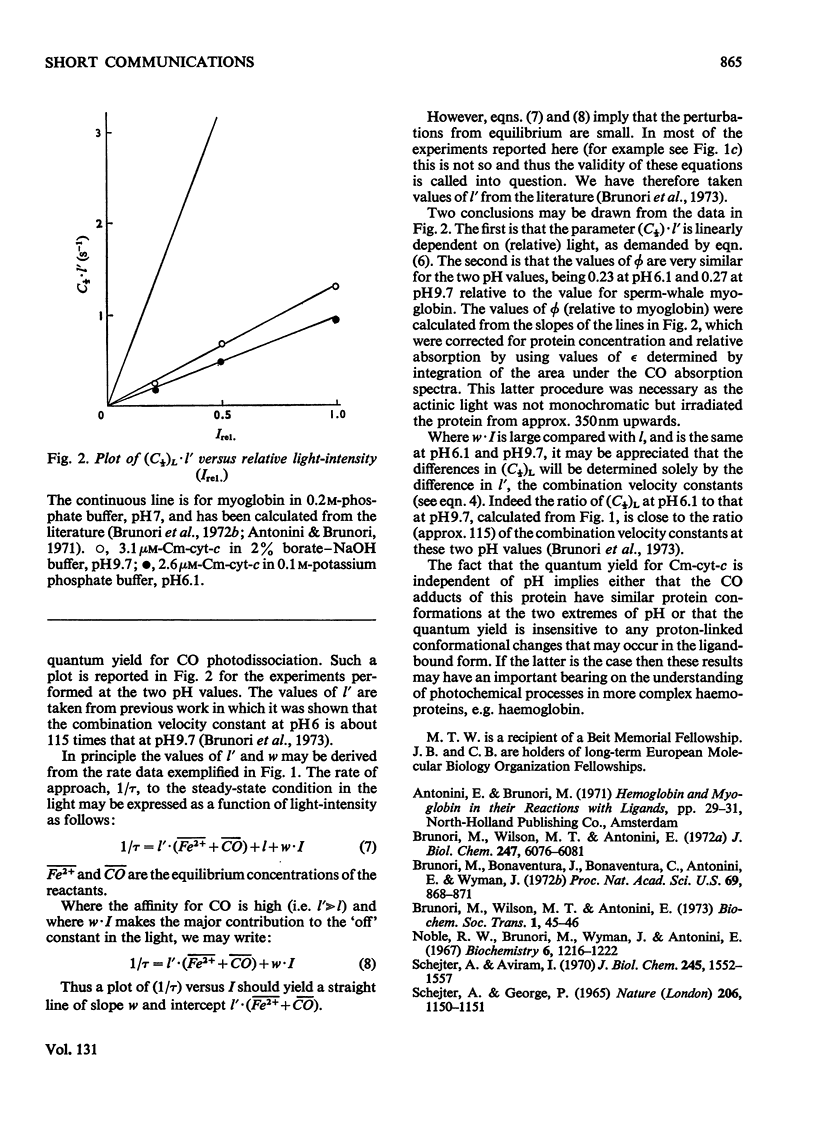
The quantum yield, [unk], is reported for the photodissociation of CO from reduced carboxymethylated cytochrome c. The values of [unk] obtained are low relative to that for myoglobin and are pH-independent, being 0.23 at pH6.1 and 0.27 at pH9.7.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunori M., Bonaventura J., Bonaventura C., Antonini E., Wyman J. Carbon monoxide binding by hemoglobin and myoglobin under photodissociating conditions. Proc Natl Acad Sci U S A. 1972 Apr;69(4):868–871. doi: 10.1073/pnas.69.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori M., Wilson M. T., Antonini E. Properties of modified cytochromes. I. Equilibrium and kinetics of the pH-dependent transition in carboxymethylated horse heart cytochrome c. J Biol Chem. 1972 Oct 10;247(19):6076–6081. [PubMed] [Google Scholar]
- Noble R. W., Brunori M., Wyman J., Antonini E. Studies on the quantum yields of the photodissociation of carbon monoxide from hemoglobin and myoglobin. Biochemistry. 1967 Apr;6(4):1216–1222. doi: 10.1021/bi00856a035. [DOI] [PubMed] [Google Scholar]
- Schejter A., Aviram I. The effects of alkylation of methionyl residues on the properties of horse cytochrome c. J Biol Chem. 1970 Apr 10;245(7):1552–1557. [PubMed] [Google Scholar]
- Schejter A., George P. Production of a "cytochrome c" with myoglobin-like properties by alkylating the cyanide complex with bromoacetate. Nature. 1965 Jun 12;206(989):1150–1151. doi: 10.1038/2061150a0. [DOI] [PubMed] [Google Scholar]


