Abstract
1. The changes in the metabolite content in the isolated perfused rat liver and in the perfusion medium were measured after loading the liver with glycerol or dihydroxyacetone. 2. Glycerol was rapidly taken up by livers from fed and starved rats; glucose, lactate and pyruvate were discharged into the medium. The [lactate]/[pyruvate] ratio in the medium rose from 10 to 30 and in the tissue from 9.6 to 36.6. 3. The most striking effects of glycerol loading were: (i) the accumulation in the liver of α-glycerophosphate, which increased from 0.13 to 8.45μmol/g at 40min; (ii) the decrease in the concentration of adenine nucleotides to 70% of the control value at 40min. 4. The Pi content of the tissue also fell, from 4.25 to 2.31μmol/g at 10min, but the sum of the phosphates measured rose from the normal value of 13.8 to 18.8μmol/g at 40min, because of an uptake of Pi from the medium. 5. Omission of phosphate from the standard perfusion medium increased the depletion of adenine nucleotides on glycerol loading. 6. Dihydroxyacetone was more rapidly metabolized than glycerol. Again glucose, lactate and pyruvate were the main products. The [lactate]/[pyruvate] ratio remained below 10. 7. Dihydroxyacetone caused an increase of the fructose 1-phosphate content from 0.23 to 0.39μmol/g at 10min. The adenine nucleotide content of the tissue was not significantly decreased by dihydroxyacetone loading. 8. The rate of removal of both glycerol and dihydroxyacetone was about 60% greater in the livers from fed than in those from starved animals. 9. The results extend previous findings by Burch et al. (1970), who administered glycerol and dihydroxyacetone intraperitoneally.
Full text
PDF
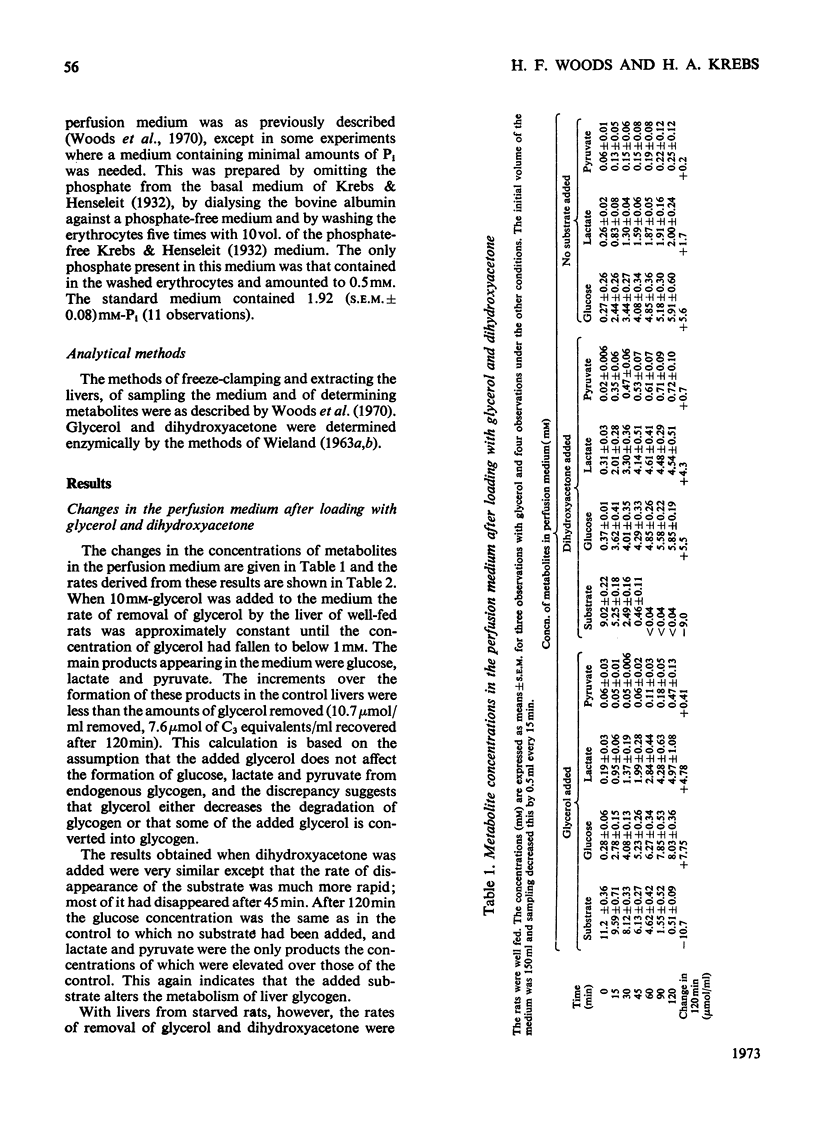
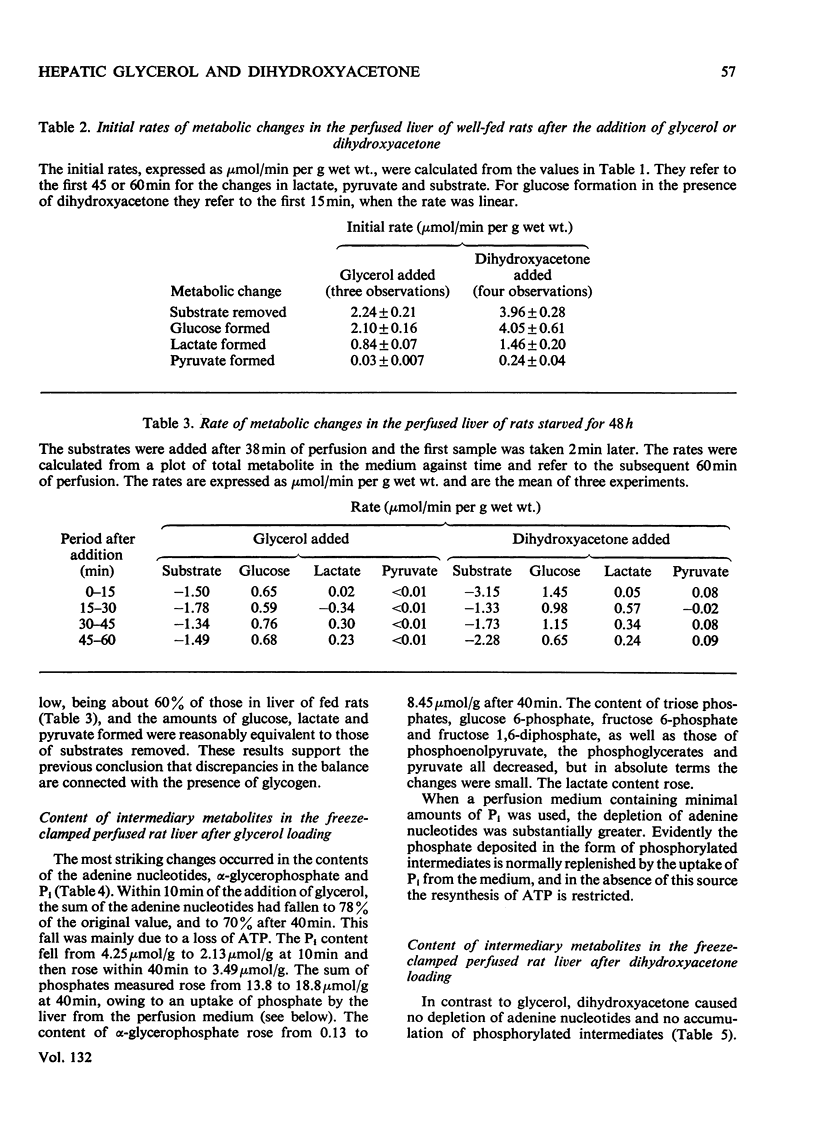
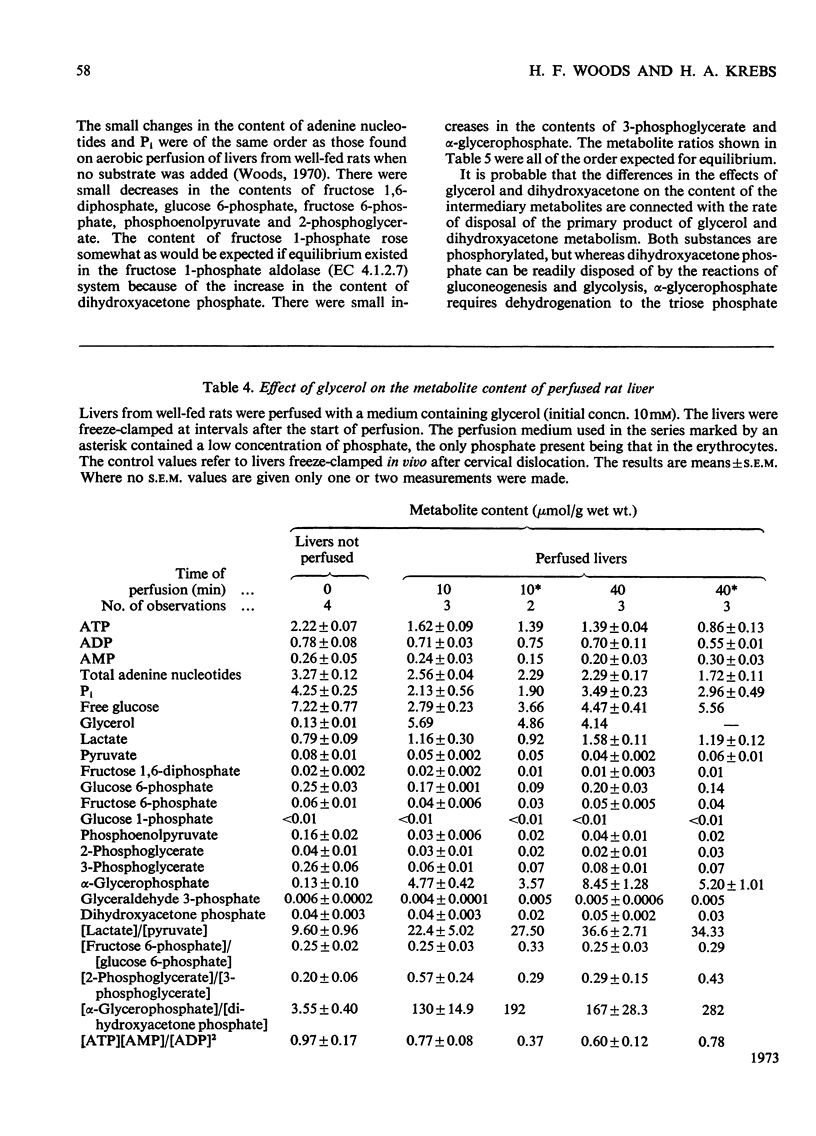


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burch H. B., Lowry O. H., Meinhardt L., Max P., Jr, Chyu K. Effect of fructose, dihydroxyacetone, glycerol, and glucose on metabolites and related compounds in liver and kidney. J Biol Chem. 1970 Apr 25;245(8):2092–2102. [PubMed] [Google Scholar]
- Burch H. B., Max P., Jr, Ghyu K., Lowry O. H. Metabolic intermediates in liver of rats given large amounts of fructose or dihydroxyacetone. Biochem Biophys Res Commun. 1969 Mar 10;34(5):619–626. doi: 10.1016/0006-291x(69)90783-9. [DOI] [PubMed] [Google Scholar]
- Carnicero H. H., Moore C. L., Hoberman H. D. Oxidation of glycerol 3-phosphate by the perfused rat liver. J Biol Chem. 1972 Jan 25;247(2):418–426. [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE Y. P., LARDY H. A. INFLUENCE OF THYROID HORMONES ON L-ALPHA-GLYCEROPHOSPHATE DEHYDROGENASES AND OTHER DEHYDROGENASES IN VARIOUS ORGANS OF THE RAT. J Biol Chem. 1965 Mar;240:1427–1436. [PubMed] [Google Scholar]
- Mäenpä P. H., Raivio K. O., Kekomäki M. P. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968 Sep 20;161(3847):1253–1254. doi: 10.1126/science.161.3847.1253. [DOI] [PubMed] [Google Scholar]
- Raivio K. O., Kekomäki M. P., Mäenpä P. H. Depletion of liver adenine nucleotides induced by D-fructose. Dose-dependence and specificity of the fructose effect. Biochem Pharmacol. 1969 Oct;18(10):2615–2624. doi: 10.1016/0006-2952(69)90192-0. [DOI] [PubMed] [Google Scholar]
- VAN EYS J., NUENKE B. J., PATTERSON M. K., Jr The nonprotein component of alpha-glycerophosphate dehydrogenase. Physical and chemical properties of the crystalline rabbit muscle enzyme. J Biol Chem. 1959 Sep;234:2308–2313. [PubMed] [Google Scholar]
- Williamson D. H., Veloso D., Ellington E. V., Krebs H. A. Changes in the concentrations of hepatic metabolites on administration of dihydroxyacetone or glycerol to starved rats and their relationship to the control of ketogenesis. Biochem J. 1969 Sep;114(3):575–584. doi: 10.1042/bj1140575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Jakob A., Refino C. Control of the removal of reducing equivalents from the cytosol in perfused rat liver. J Biol Chem. 1971 Dec 25;246(24):7632–7641. [PubMed] [Google Scholar]
- Woods H. F., Eggleston L. V., Krebs H. A. The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J. 1970 Sep;119(3):501–510. doi: 10.1042/bj1190501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG H. L., PACE N. Some physical and chemical properties of crystalline alpha-glycerophosphate dehydrogenase. Arch Biochem Biophys. 1958 May;75(1):125–141. doi: 10.1016/0003-9861(58)90403-x. [DOI] [PubMed] [Google Scholar]


