Abstract
1. The effects of teichoic acids on the Mg2+-requirement of some membrane-bound enzymes in cell preparations from Bacillus licheniformis A.T.C.C. 9945 were examined. 2. The biosynthesis of the wall polymers poly(glycerol phosphate glucose) and poly(glycerol phosphate) by membrane-bound enzymes is strongly dependent on Mg2+, showing maximum activity at 10–15mm-Mg2+. 3. When the membrane is in close contact with the cell wall and membrane teichoic acid, the enzyme systems are insensitive to added Mg2+. The membrane appears to interact preferentially with the constant concentration of Mg2+ that is bound to the phosphate groups of teichoic acid in the wall and on the membrane. When the wall is removed by the action of lysozyme the enzymes again become dependent on an external supply of Mg2+. 4. A membrane preparation that retained its membrane teichoic acid was still dependent on Mg2+ in solution, but the dependence was damped so that the enzymes exhibited near-maximal activity over a much greater range of concentrations of added Mg2+; this preparation contained Mg2+ bound to the membrane teichoic acid. The behaviour of this preparation could be reproduced by binding membrane teichoic acid to membranes in the presence of Mg2+. Addition of membrane teichoic acid to reaction mixtures also had a damping effect on the Mg2+ requirement of the enzymes, since the added polymer interacted rapidly with the membrane. 5. Other phosphate polymers behaved in a qualitatively similar way to membrane teichoic acid on addition to reaction mixtures. 6. It is concluded that in whole cells the ordered array of anionic wall and membrane teichoic acids provides a constant reservoir of bound bivalent cations with which the membrane preferentially interacts. The membrane teichoic acid is the component of the system which mediates the interaction of bound cations with the membrane. The anionic polymers in the wall scavenge cations from the medium and maintain a constant environment for the membrane teichoic acid. Thus a function of wall and membrane teichoic acids is to maintain the correct ionic environment for cation-dependent membrane systems.
Full text
PDF




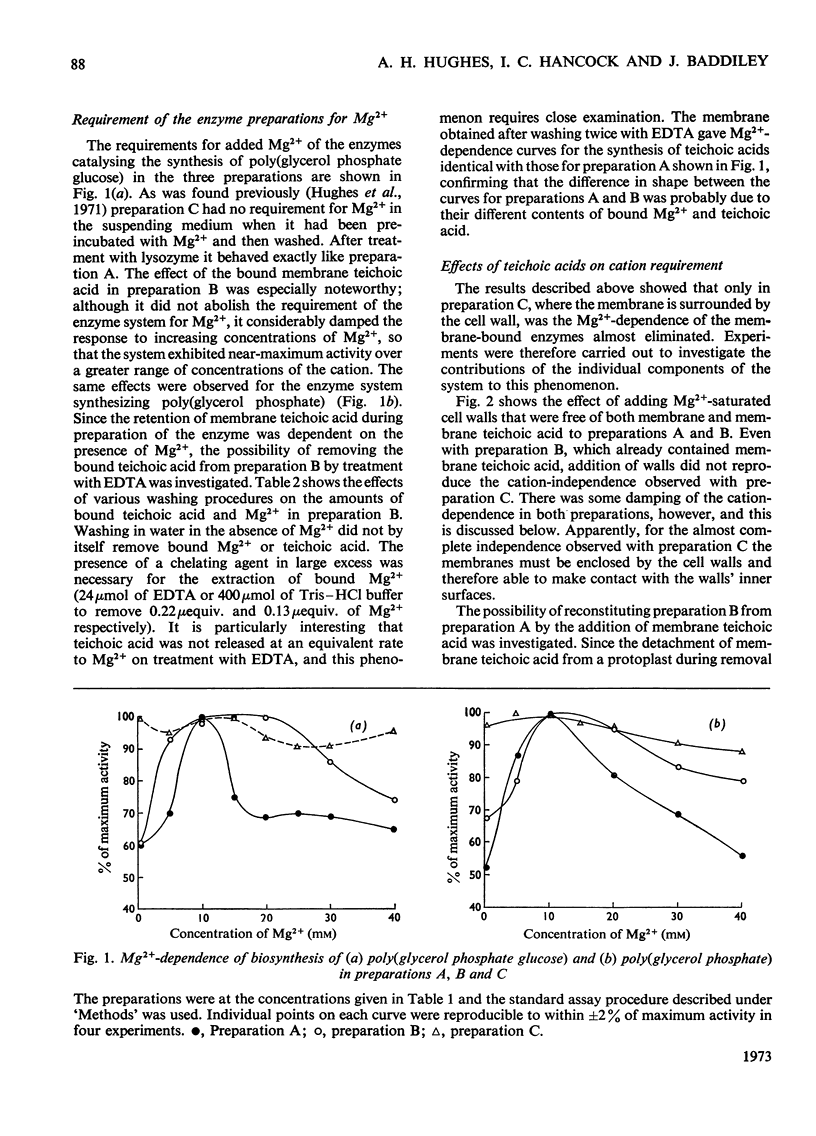
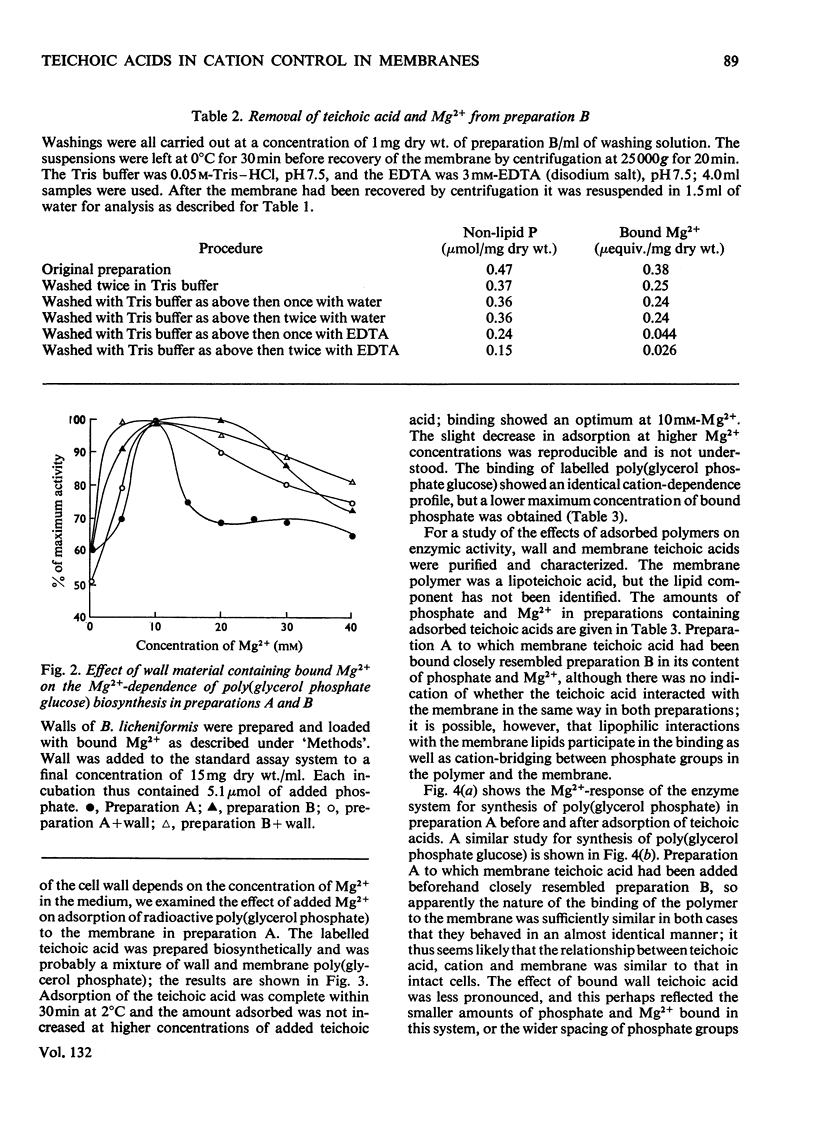
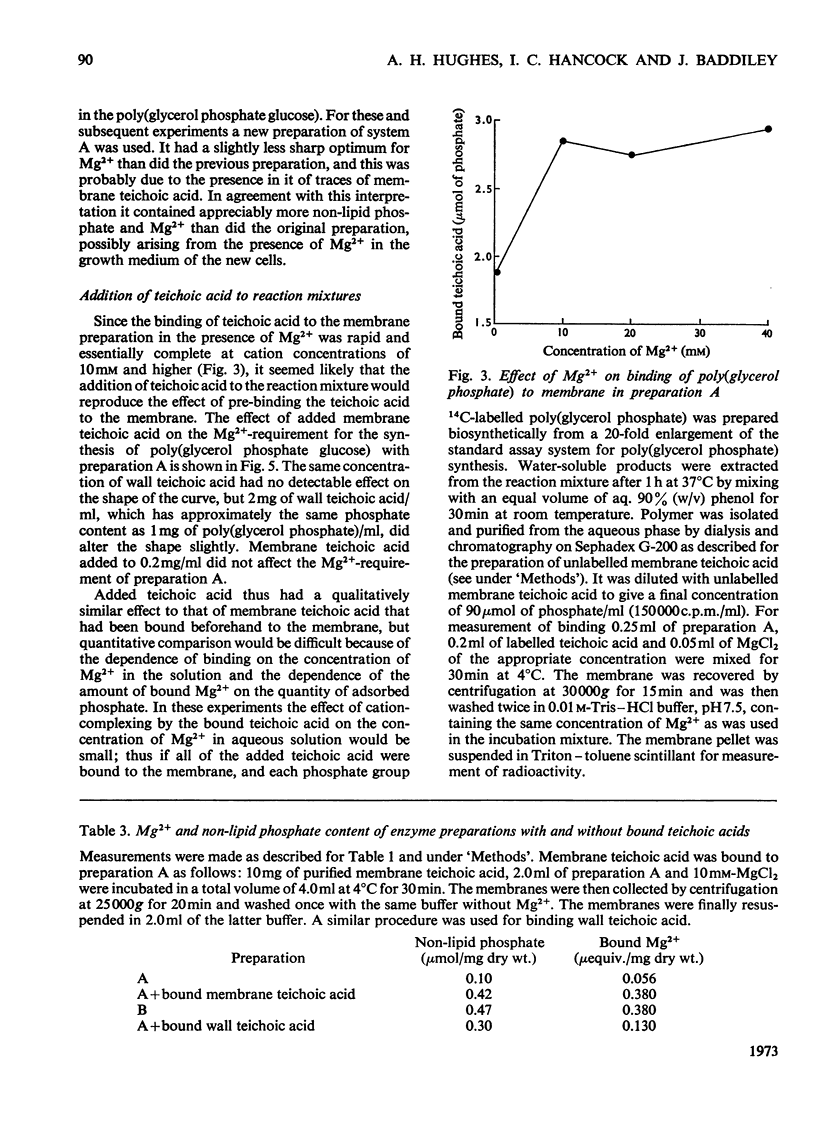



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Hussey H., Baddiley J. The mechanism of wall synthesis in bacteria. The organization of enzymes and isoprenoid phosphates in the membrane. Biochem J. 1972 Mar;127(1):11–25. doi: 10.1042/bj1270011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald A. R., Baddiley J. The teichoic acids. Adv Carbohydr Chem Biochem. 1966;21:323–375. doi: 10.1016/s0096-5332(08)60320-3. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BURGER M. M., GLASER L. THE SYNTHESIS OF TEICHOIC ACIDS. I. POLYGLYCEROPHOSPHATE. J Biol Chem. 1964 Oct;239:3168–3177. [PubMed] [Google Scholar]
- Baddiley J., Blumsom N. L., Douglas L. J. The biosynthesis of the wall teichoic acid in Staphylococcus lactis I3. Biochem J. 1968 Dec;110(3):565–571. doi: 10.1042/bj1100565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddiley J. Teichoic acids in cell walls and membranes of bacteria. Essays Biochem. 1972;8:35–77. [PubMed] [Google Scholar]
- Burger M. M., Glaser L. The synthesis of teichoic acids. V. Polyglucosylglycerol phosphate and polygalactosylglycerol phosphate. J Biol Chem. 1966 Jan 25;241(2):494–506. [PubMed] [Google Scholar]
- Cole H. A., Hughes D. E. The enzymic activity of the outer shell of Lactobacillus arabinosus. J Gen Microbiol. 1965 Jul;40(1):81–95. doi: 10.1099/00221287-40-1-81. [DOI] [PubMed] [Google Scholar]
- FRANCIS M. J., HUGHES D. E., KORNBERG H. L., PHIZACKERLEY P. J. THE OXIDATION OF L-MALATE BY PSEUDOMONAS SP. Biochem J. 1963 Dec;89:430–438. doi: 10.1042/bj0890430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMMES G. G., LEVISON S. A. A KINETIC INVESTIGATION OF THE INTERACTION OF ADENOSINE-5'-TRIPHOSPHATE WITH DIVALENT METAL IONS. Biochemistry. 1964 Oct;3:1504–1506. doi: 10.1021/bi00898a019. [DOI] [PubMed] [Google Scholar]
- HAY J. B., WICKEN A. J., BADDILEY J. The location of intracellular teichoic acids. Biochim Biophys Acta. 1963 Apr 2;71:188–190. doi: 10.1016/0006-3002(63)90999-5. [DOI] [PubMed] [Google Scholar]
- HUNT A. L., RODGERS A., HUGHES D. E. Sub-cellular particles and the nicotinic acid hydroxylase system in extracts of. Biochim Biophys Acta. 1959 Aug;34:354–372. doi: 10.1016/0006-3002(59)90288-4. [DOI] [PubMed] [Google Scholar]
- Hancock I. C., Baddiley J. Biosynthesis of the wall teichoic acid in Bacillus licheniformis. Biochem J. 1972 Mar;127(1):27–37. doi: 10.1042/bj1270027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptinstall S., Archibald A. R., Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970 Feb 7;225(5232):519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
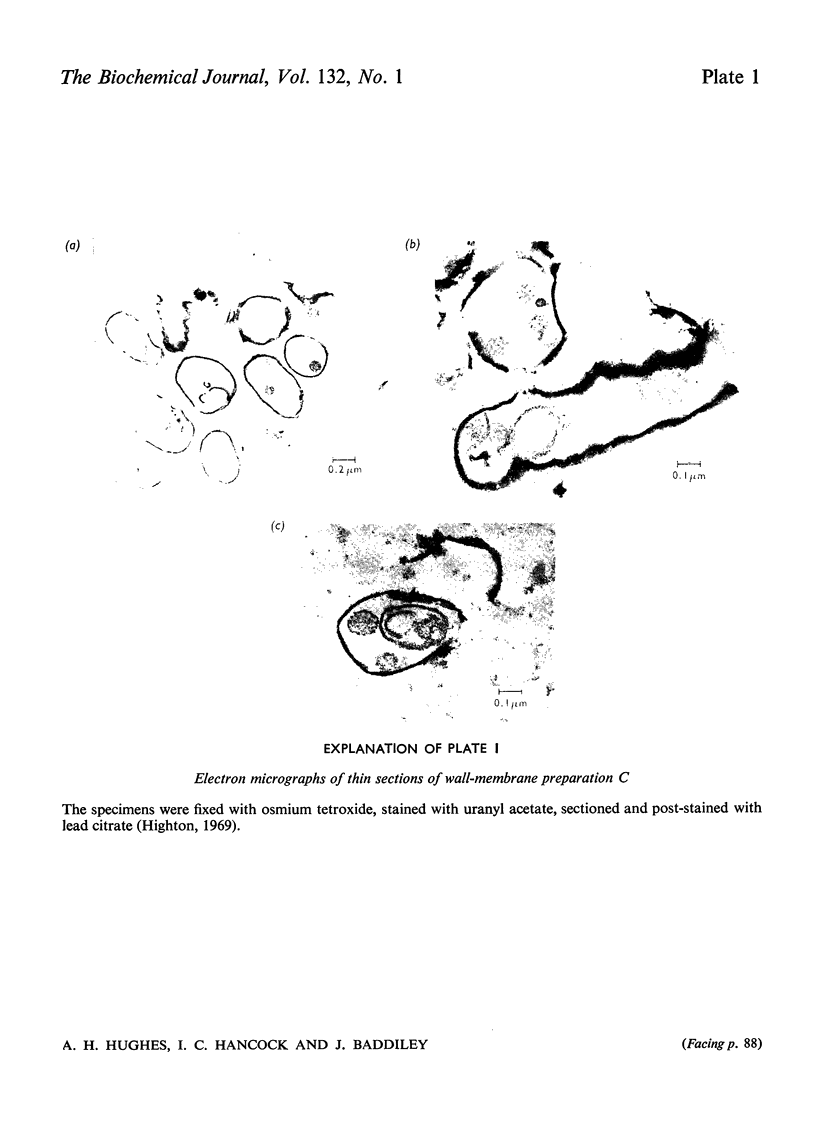
- Highton P. J. An electron microscopic study of cell growth and mesosomal structure of Bacillus licheniformis. J Ultrastruct Res. 1969 Jan;26(1):130–147. doi: 10.1016/s0022-5320(69)90040-9. [DOI] [PubMed] [Google Scholar]
- Hughes A. H., Stow M., Hancock I. C., Baddiley J. Function of teichoic acids and effect of novobiocin on control of Mg2+ at the bacterial membrane. Nat New Biol. 1971 Jan 13;229(2):53–55. doi: 10.1038/newbio229053a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lastras M., Muñoz E. Properties of the membrane-adenosine triphosphatase complex of Micrococcus lysodeikticus: Reversibility of the Mg(2+)-dependent states of the ATPase. FEBS Lett. 1972 Mar 15;21(2):109–112. doi: 10.1016/0014-5793(72)80115-7. [DOI] [PubMed] [Google Scholar]
- PALADINI A. C., LELOIR L. F. Studies on uridine-diphosphate-glucose. Biochem J. 1952 Jun;51(3):426–430. doi: 10.1042/bj0510426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H., Lennarz W. J. Studies on the membranes of bacilli. I. Phospholipid biosynthesis. J Biol Chem. 1971 Feb 25;246(4):1062–1072. [PubMed] [Google Scholar]
- Reaveley D. A., Rogers H. J. Some enzymic activities and chemical properties of the mesosomes and cytoplasmic membranes of Bacillus licheniformis 6346. Biochem J. 1969 Jun;113(1):67–79. doi: 10.1042/bj1130067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Dicks J. W., Ellwood D. C. Influence of growth condition on the concentration of potassium in Bacillus subtilis var. niger and its possible relationship to cellular ribonucleic acid, teichoic acid and teichuronic acid. Biochem J. 1968 Jan;106(1):237–243. doi: 10.1042/bj1060237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toon P., Brown P. E., Baddiley J. The lipid-teichoic acid complex in the cytoplasmic membrane of Streptococcus faecalis N.C.I.B. 8191. Biochem J. 1972 Apr;127(2):399–409. doi: 10.1042/bj1270399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J Gen Microbiol. 1970 Mar;60(3):293–301. doi: 10.1099/00221287-60-3-293. [DOI] [PubMed] [Google Scholar]



