Abstract
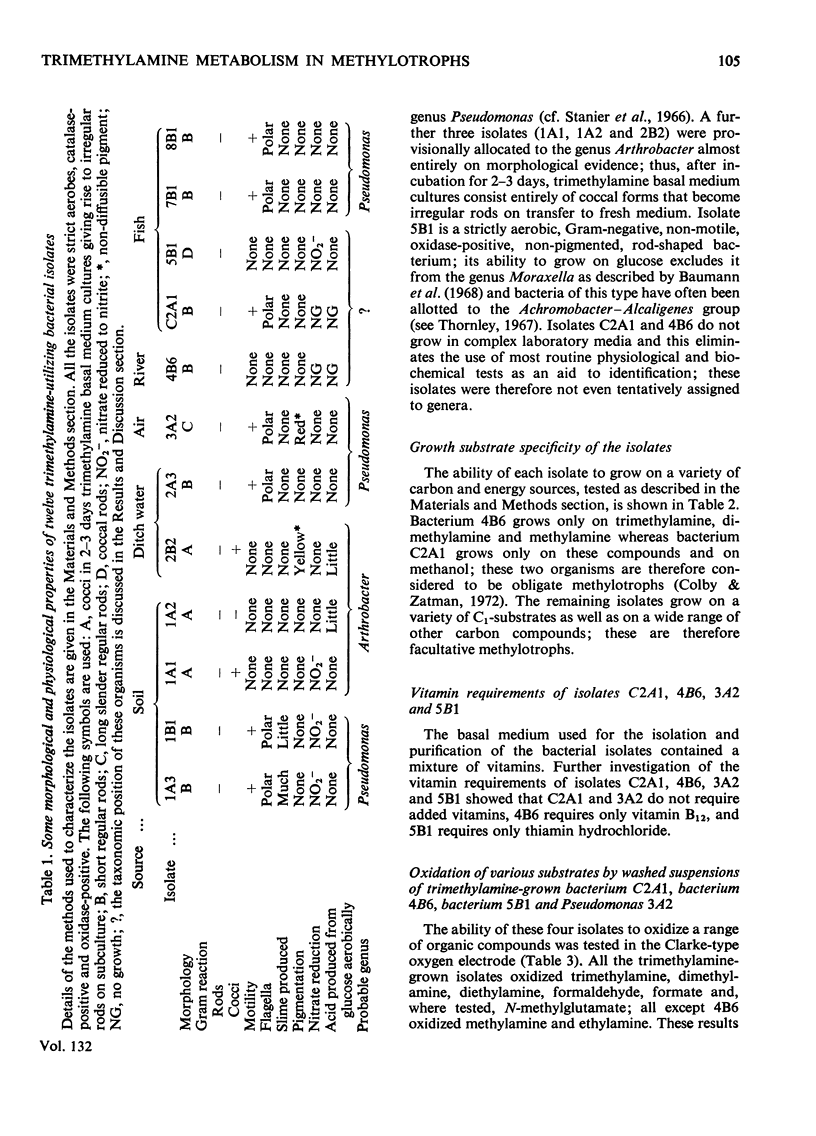
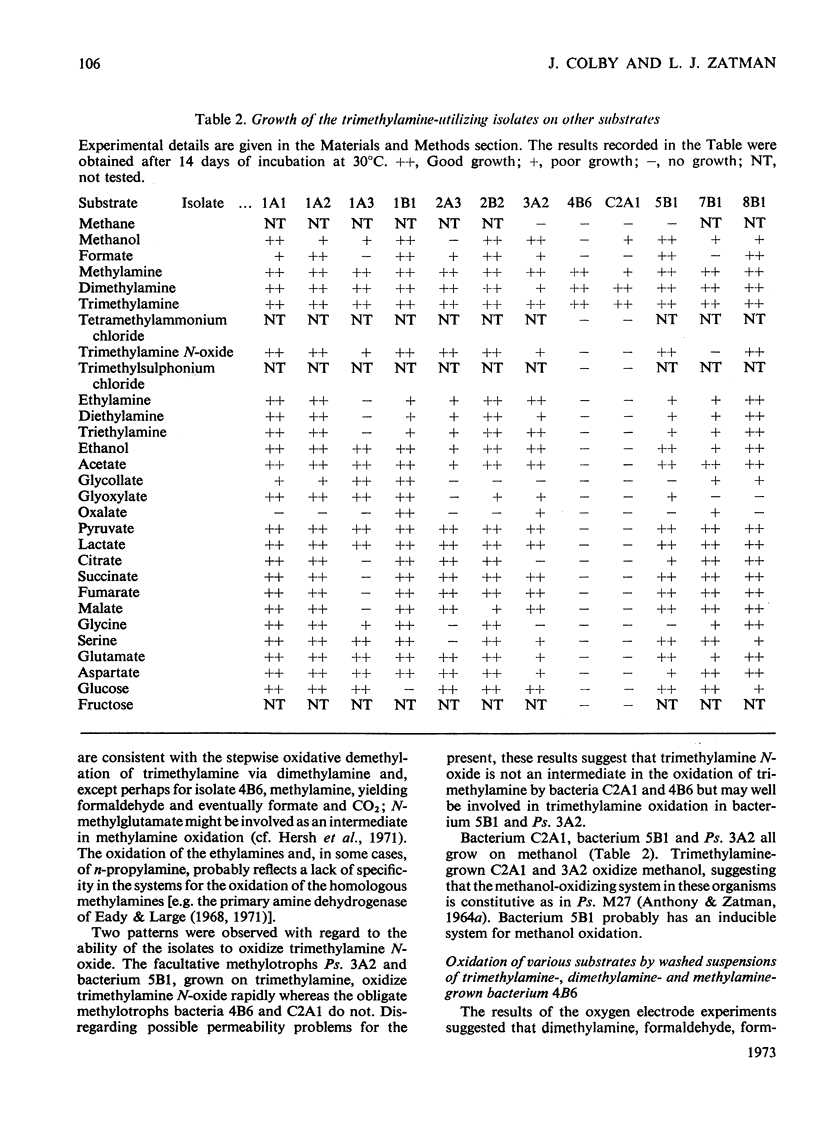
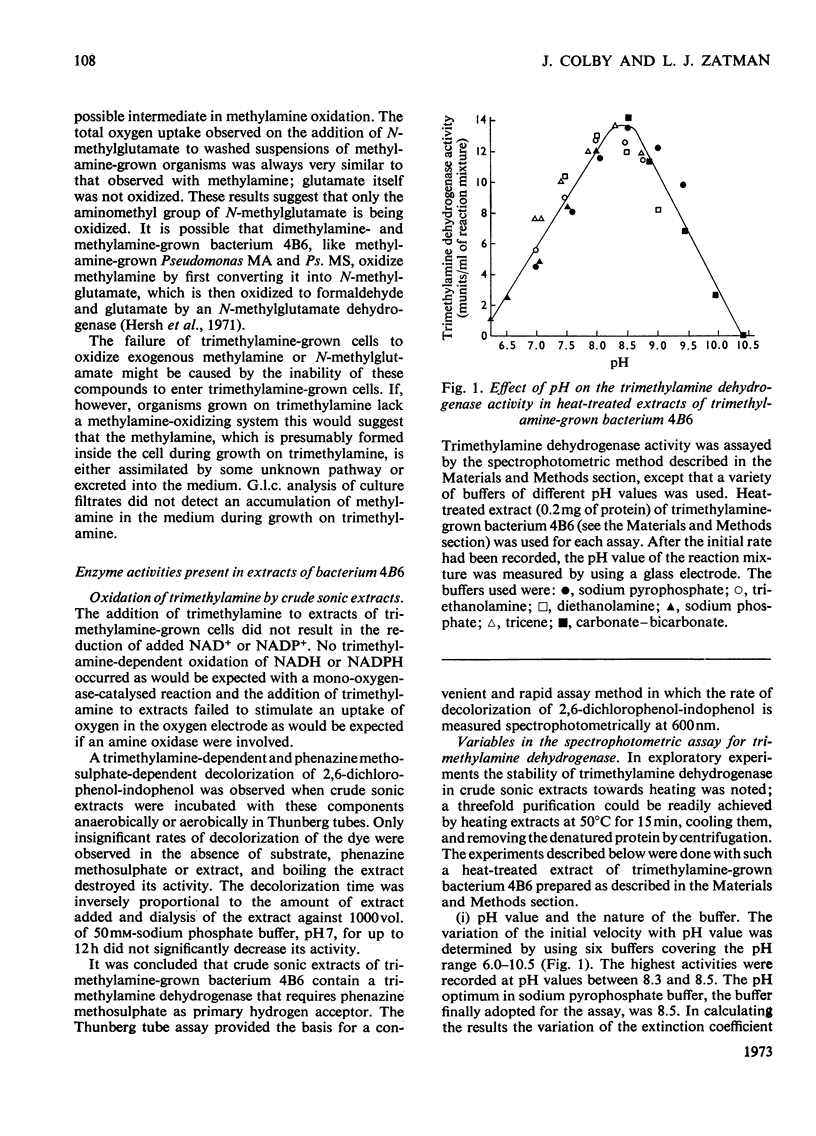
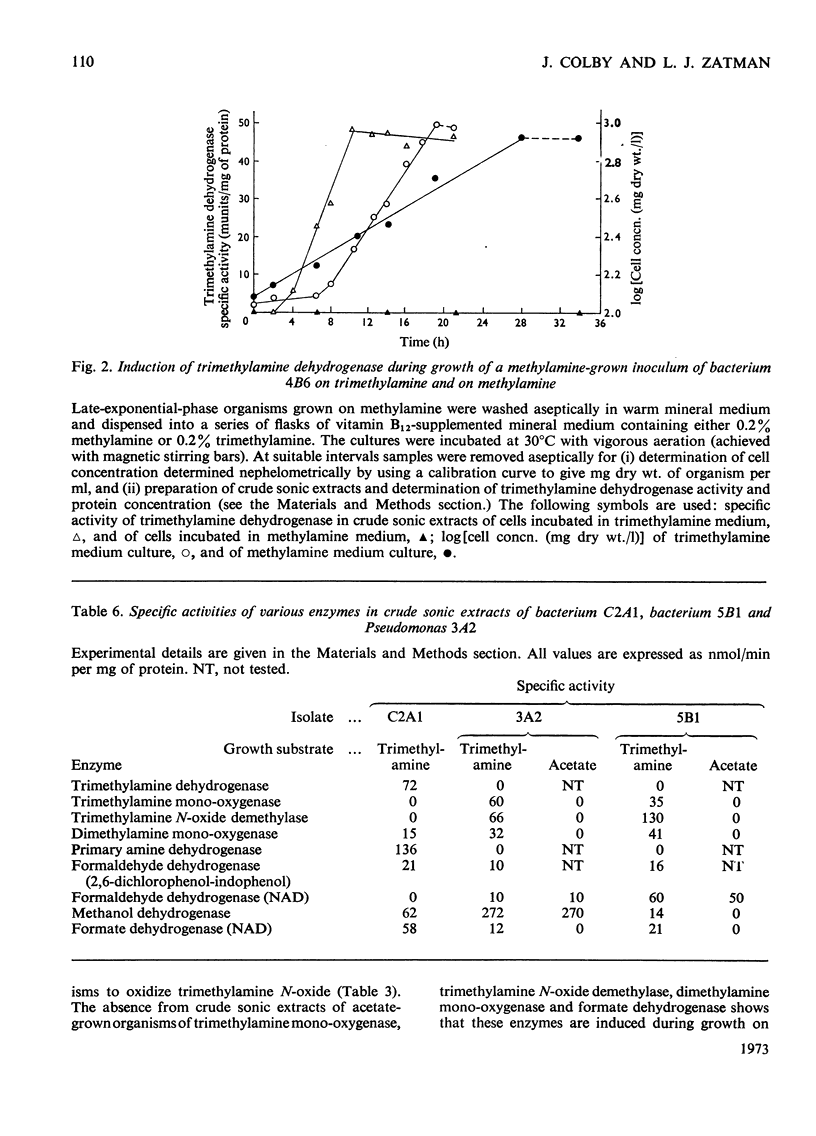
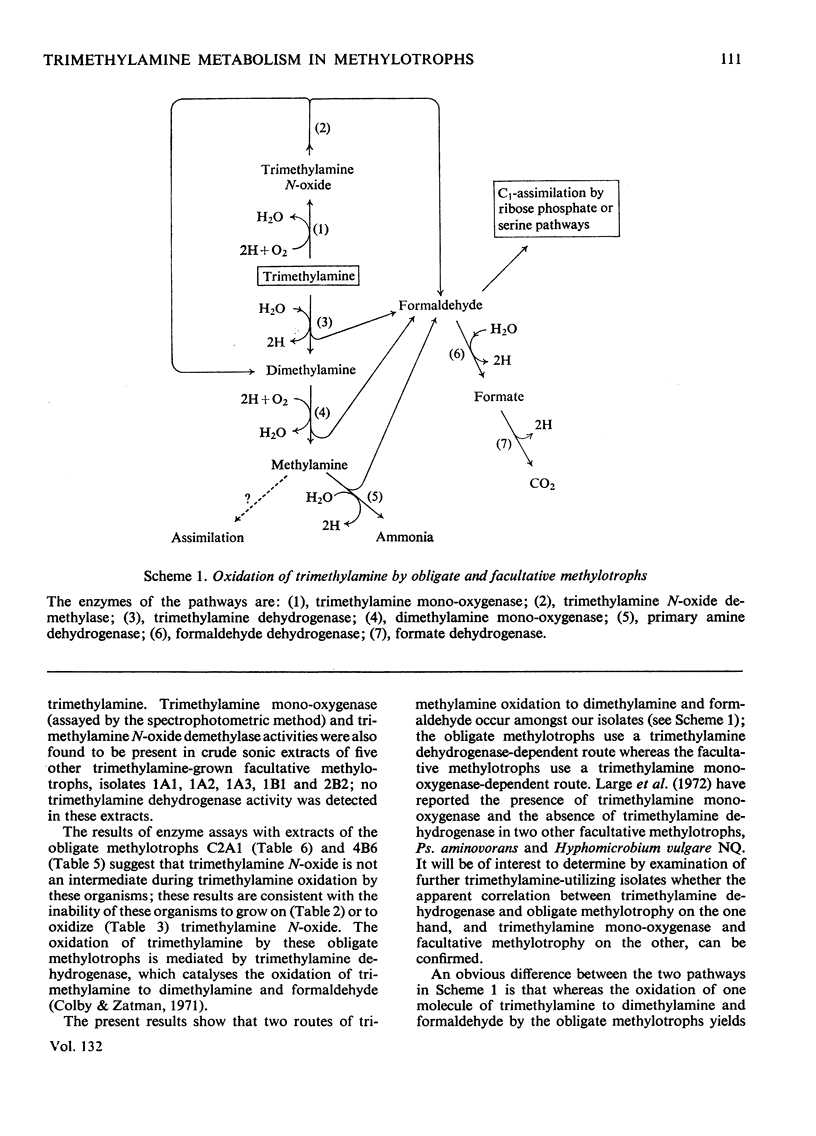
1. Twelve bacterial isolates that grow with trimethylamine as sole source of carbon and energy were obtained in pure culture. All the isolates grow on methylamine, dimethylamine and trimethylamine. One isolate, bacterium 4B6, grows only on these methylamines whereas another isolate, bacterium C2A1, also grows on methanol but neither grows on methane; these two organisms are obligate methylotrophs. The other ten isolates grow on a variety of Ci and other organic compounds and are therefore facultative methylotrophs. 2. Washed suspensions of the obligate methylotrophs bacteria 4B6 and C2A1, and of the facultative methylotrophs bacterium 5B1 and Pseudomonas 3A2, all grown on trimethylamine, oxidize trimethylamine, dimethylamine, formaldehyde and formate; only bacterium 5B1 and Ps. 3A2 oxidize trimethylamine N-oxide; only bacterium 4B6 does not oxidize methylamine. 3. Cell-free extracts of trimethylamine-grown bacteria 4B6 and C2A1 contain a trimethylamine dehydrogenase that requires phenazine methosulphate as primary hydrogen acceptor, and evidence is presented that this enzyme is important for the growth of bacterium 4B6 on trimethylamine. 4. Cell-free extracts of eight facultative methylotrophs, including bacterium 5B1 and Ps. 3A2, do not contain trimethylamine dehydrogenase but contain instead a trimethylamine monooxygenase and trimethylamine N-oxide demethylase. It is concluded that two different pathways for the oxidation of trimethylamine occur amongst the isolates.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. M. THE MOLAR EXTINCTION COEFFICIENT OF 2,6-DICHLOROPHENOL INDOPHENOL. Biochim Biophys Acta. 1964 Apr 4;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. 1. Isolation and properties of Pseudomonas sp. M27. Biochem J. 1964 Sep;92(3):609–614. doi: 10.1042/bj0920609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. 2. The methanol-oxidizing enzyme of Pseudomonas sp. M 27. Biochem J. 1964 Sep;92(3):614–621. doi: 10.1042/bj0920614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER J. R., CHAYKIN S. The biosynthesis of trimethylamine-N-oxide. J Biol Chem. 1962 Apr;237:1309–1313. [PubMed] [Google Scholar]
- BAKER J. R., STRUEMPLER A., CHAYKIN S. A comparative study of trimethylamine-N-oxide biosynthesis. Biochim Biophys Acta. 1963 Apr 2;71:58–64. doi: 10.1016/0006-3002(63)90985-5. [DOI] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. Study of the Moraxella group. I. Genus Moraxella and the Neisseria catarrhalis group. J Bacteriol. 1968 Jan;95(1):58–73. doi: 10.1128/jb.95.1.58-73.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Zatman L. J. Hexose phosphate synthese and tricarboxylic acid-cycle enzymes in bacterium 4B6, an obligate methylotroph. Biochem J. 1972 Aug;128(5):1373–1376. doi: 10.1042/bj1281373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Jarman T. R., Large P. J. Microbial oxidation of amines. Partial purification of a mixed-function secondary-amine oxidase system from Pseudomonas aminovorans that contains an enzymically active cytochrome-P-420-type haemoprotein. Biochem J. 1971 Nov;125(2):449–459. doi: 10.1042/bj1250449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Microbial oxidation of amines. Spectral and kinetic properties of the primary amine dehydrogenase of Pseudomonas AM1. Biochem J. 1971 Aug;123(5):757–771. doi: 10.1042/bj1230757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Purification and properties of an amine dehydrogenase from Pseudomonas AM1 and its role in growth on methylamine. Biochem J. 1968 Jan;106(1):245–255. doi: 10.1042/bj1060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- HUGH R., LEIFSON E. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J Bacteriol. 1953 Jul;66(1):24–26. doi: 10.1128/jb.66.1.24-26.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh L. B., Peterson J. A., Thompson A. A. An N-methyl glutamate dehydrogenase from Pseudomonas M.A. Arch Biochem Biophys. 1971 Jul;145(1):115–120. doi: 10.1016/0003-9861(71)90016-6. [DOI] [PubMed] [Google Scholar]
- JOHNSON P. A., JONES-MORTIMER M. C., QUAYLE J. R. USE OF A PURIFIED BACTERIAL FORMATE DEHYDROGENASE FOR THE MICRO-ESTIMATION OF FORMATE. Biochim Biophys Acta. 1964 Aug 26;89:351–353. doi: 10.1016/0926-6569(64)90225-1. [DOI] [PubMed] [Google Scholar]
- Johnson P. A., Quayle J. R. Microbial growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM-1. Biochem J. 1964 Nov;93(2):281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Enzymes of the mandelate pathway in Bacterium N.C.I.B. 8250. Biochem J. 1968 Apr;107(4):497–506. doi: 10.1042/bj1070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIFSON E. Staining, shape and arrangement of bacterial flagella. J Bacteriol. 1951 Oct;62(4):377–389. doi: 10.1128/jb.62.4.377-389.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large P. J. Non-oxidative demethylation of trimethylamine N-oxide by Pseudomonas aminovorans. FEBS Lett. 1971 Nov 1;18(2):297–300. doi: 10.1016/0014-5793(71)80470-2. [DOI] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J. D., Keddie R. M. The nitrogen nutrition of soil and herbage coryneform bacteria. J Appl Bacteriol. 1969 Sep;32(3):338–347. doi: 10.1111/j.1365-2672.1969.tb00981.x. [DOI] [PubMed] [Google Scholar]
- RHODES M. E. The cytology of Pseudomonas spp. as revealed by a silver-plating staining method. J Gen Microbiol. 1958 Jun;18(3):639–648. doi: 10.1099/00221287-18-3-639. [DOI] [PubMed] [Google Scholar]
- ROSETT T. COOLING DEVICE FOR USE WITH A SONIC OSCILLATOR. Appl Microbiol. 1965 Mar;13:254–256. doi: 10.1128/am.13.2.254-256.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons D. W., Harrison J. E., Wadzinski A. M. Metabolism of single carbon compounds. Annu Rev Microbiol. 1970;24:135–158. doi: 10.1146/annurev.mi.24.100170.001031. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Thornley M. J. A taxonomic study of Acinetobacter and related genera. J Gen Microbiol. 1967 Nov;49(2):211–257. doi: 10.1099/00221287-49-2-211. [DOI] [PubMed] [Google Scholar]
- Whittenbury R., Phillips K. C., Wilkinson J. F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]


