Abstract
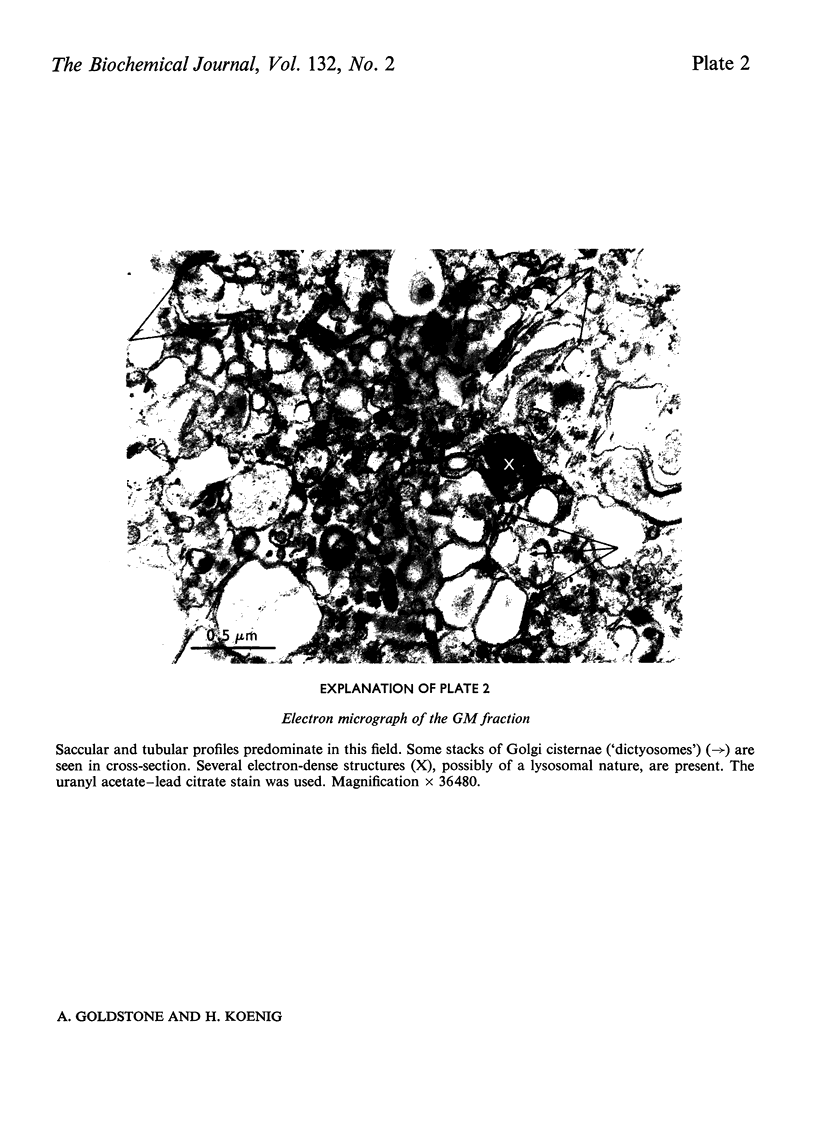
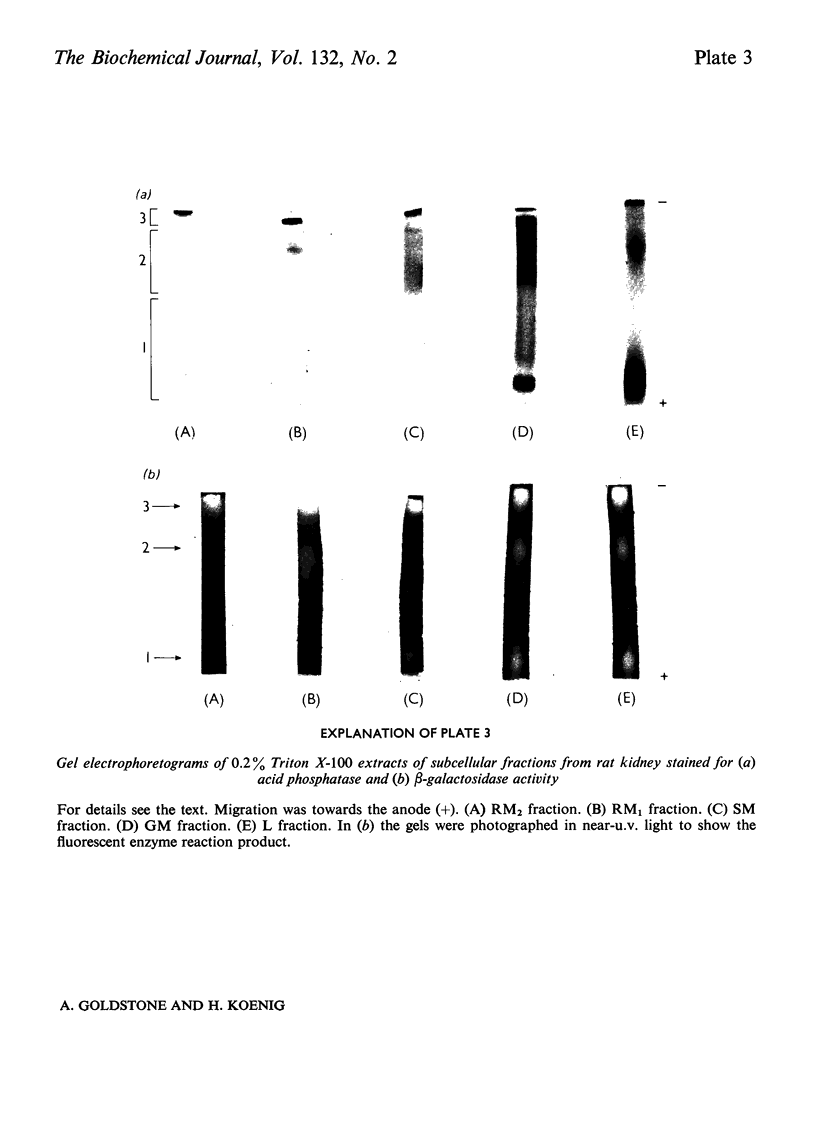
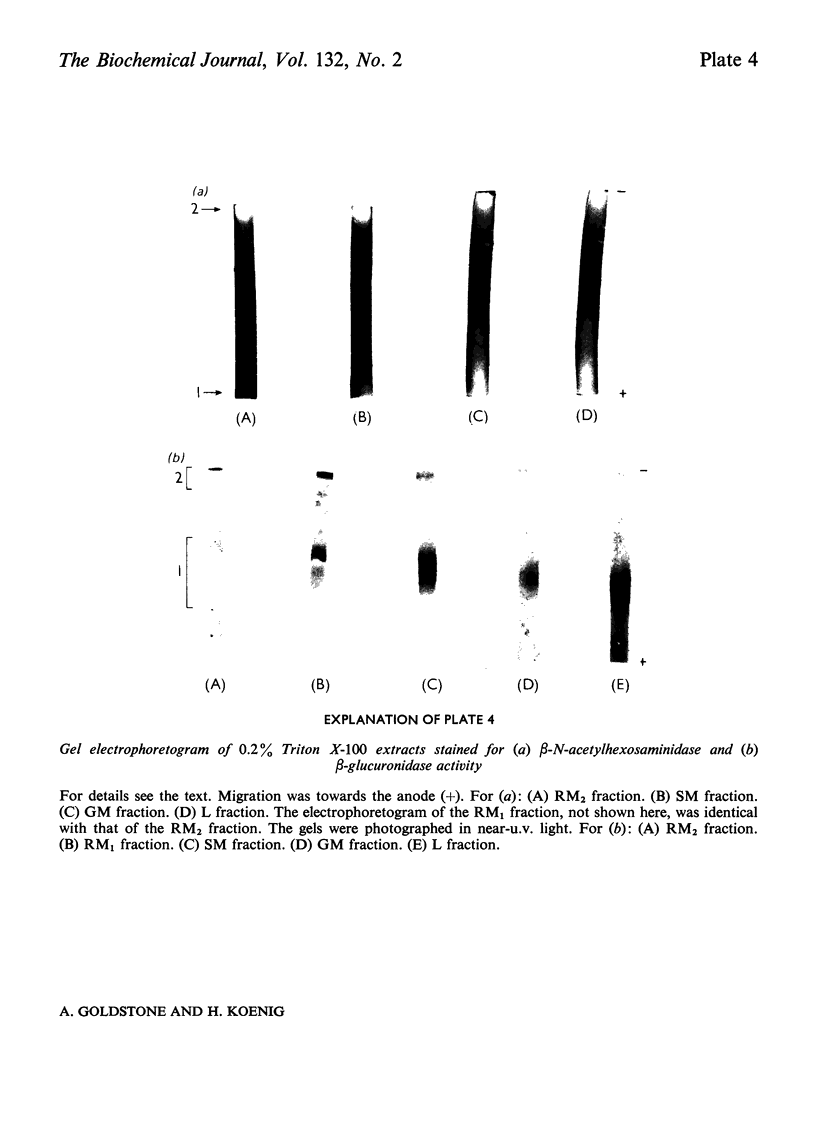
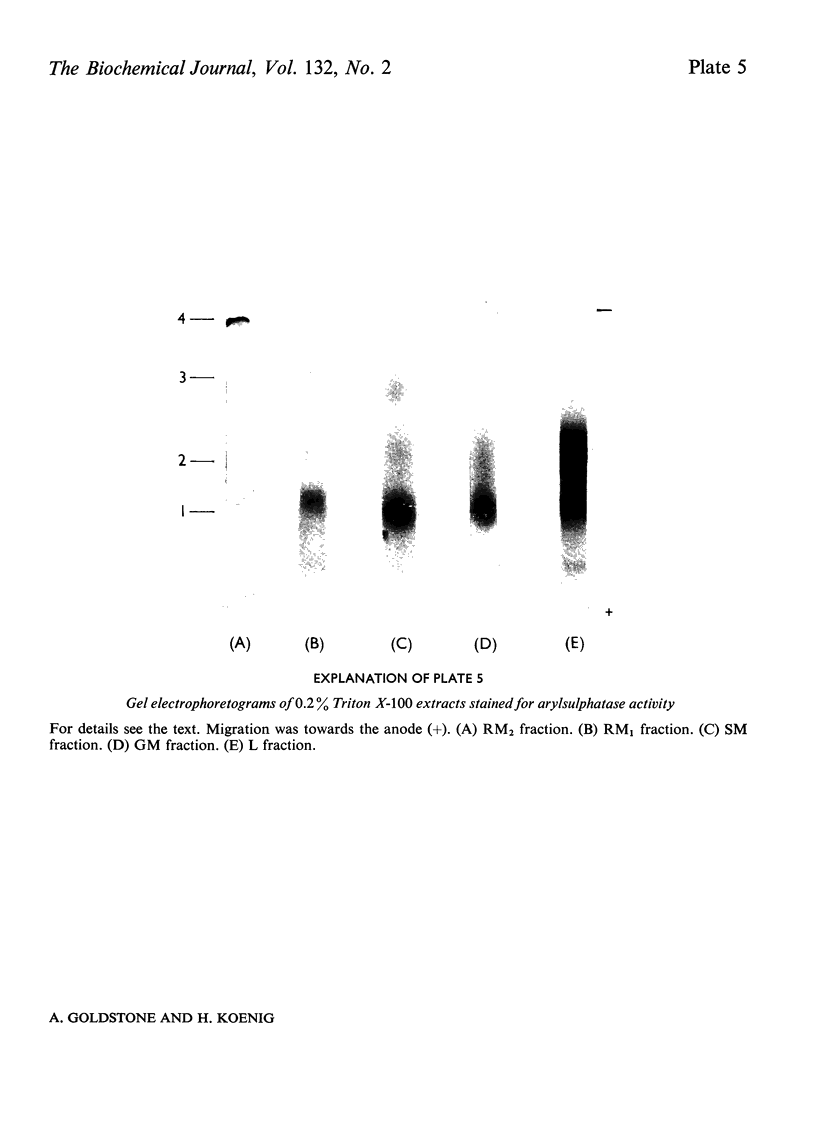
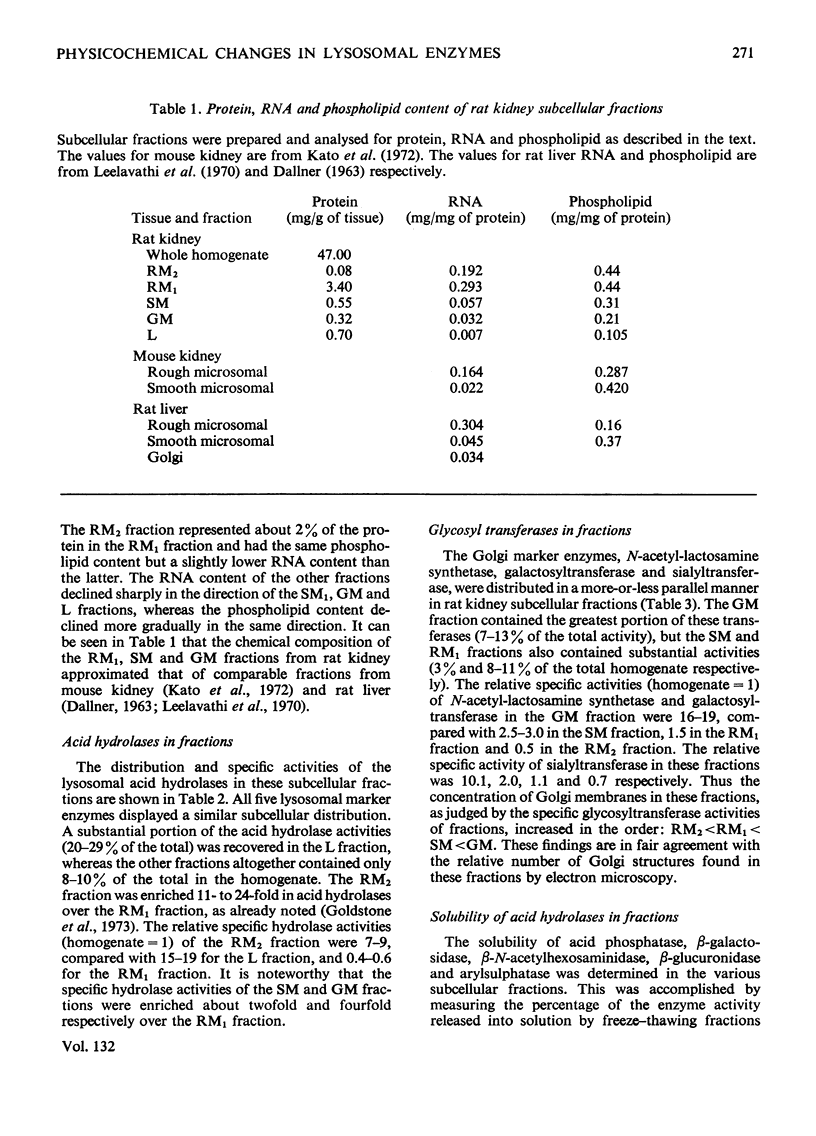
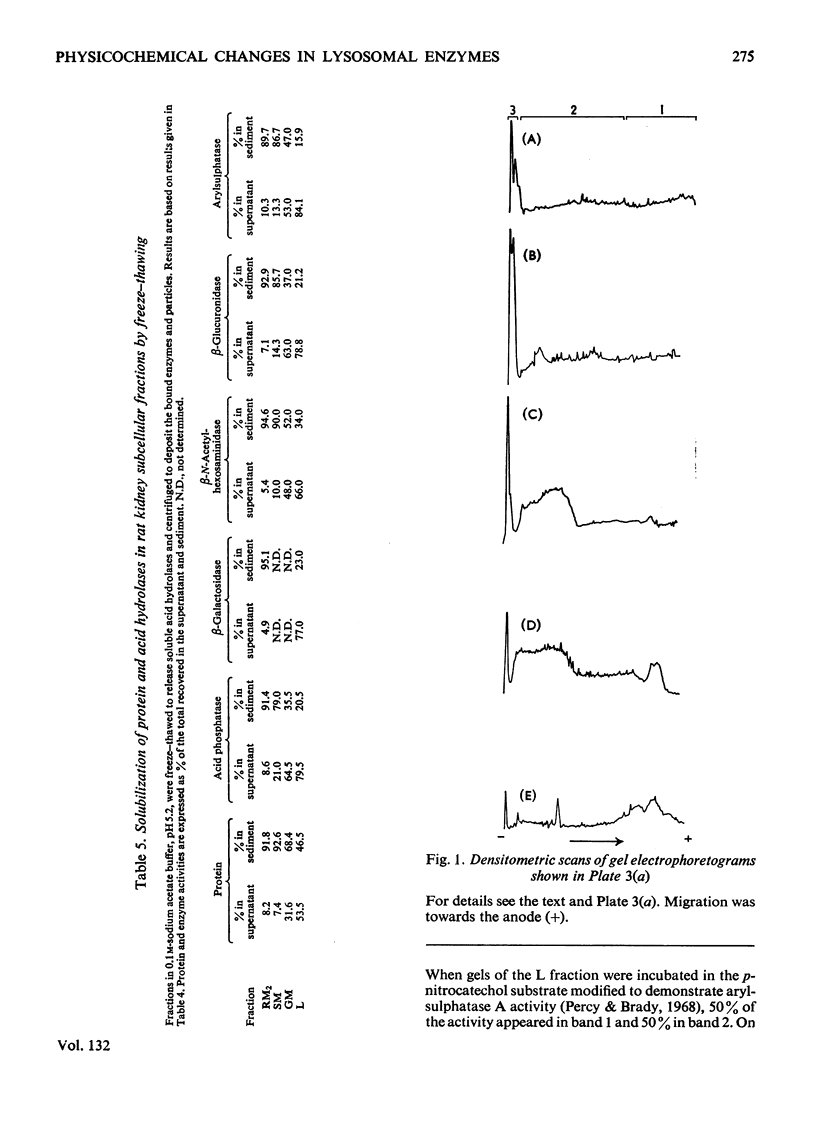
1. The following fractions were prepared from rat kidney and characterized ultrastructurally, biochemically and enzymically: (a) an ordinary rough microsomal (RM1) fraction; (b) a special rough microsomal (RM2) fraction enriched seven- to nine-fold in acid hydrolases over the homogenate; (c) a smooth microsomal (SM) fraction; (d) a Golgi (GM) fraction enriched 2.5-fold in acid hydrolases and 10-, 15- and 20-fold in sialyltransferase, N-acetyl-lactosamine synthetase and galactosyltransferase respectively; (e) a lysosomal (L) fraction enriched 15- to 23-fold in acid hydrolases. The frequency of Golgi sacs and tubules seen in the electron microscope and the specific activity of the three glycosyltransferases in these fractions increased in the order: RM2<RM1<SM<GM. 2. Five lysosomal hydrolases, acid phosphatase, β-N-acetyl-hexosaminidase, β-galactosidase, β-glucuronidase and arylsulphatase, were characterized in these fractions with respect to (a) solubility on freeze–thawing and (b) electrophoretic mobility in polyacrylamide gels. 3. In the RM2 fraction each of these hydrolases occurred largely or exclusively as a single bound basic form coincident with cationic glycoprotein bands in gels (Goldstone et al., 1973). 4. In the L fraction these hydrolases were present largely as soluble, acidic (anionic) forms. 5. The solubility, electrophoretic heterogeneity and anodic mobility of these hydrolases increased progressively in subcellular fractions in the order: RM2<RM1<SM<GM<L. 6. These findings, together with evidence cited in the text showing that N-acetylneuraminic acid residues are responsible for the solubility and electronegative charge of these acidic forms and incorporation of these residues into the Golgi apparatus, support the following scheme for the biosynthesis of lysosomal enzymes. Each hydrolase is synthesized as a bound basic glycoprotein enzyme in a restricted portion of the rough endoplasmic reticulum. The soluble, acidic forms are generated as the nascent glycoprotein enzymes migrate through the Golgi apparatus through the attachment of sugar sequences containing N-acetylneuraminic acid.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J. The effect of autolysis on the distribution of acid phosphatase in rat brain. J Neurochem. 1965 Nov;12(11):919–925. doi: 10.1111/j.1471-4159.1965.tb11935.x. [DOI] [PubMed] [Google Scholar]
- Austin J., Armstrong D., Shearer L. Metachromatic form of diffuse cerebral sclerosis. V. The nature and significance of low sulfatase activity: a controlled study of brain, liver and kidney in four patients with metachromatic leukodystrophy (MLD). Arch Neurol. 1965 Dec;13(6):593–614. doi: 10.1001/archneur.1965.00470060029003. [DOI] [PubMed] [Google Scholar]
- BARKA T. Studies on acid phosphatases. II. Chromatographic separation of acid phosphatases of rat liver. J Histochem Cytochem. 1961 Sep;9:564–571. doi: 10.1177/9.5.564. [DOI] [PubMed] [Google Scholar]
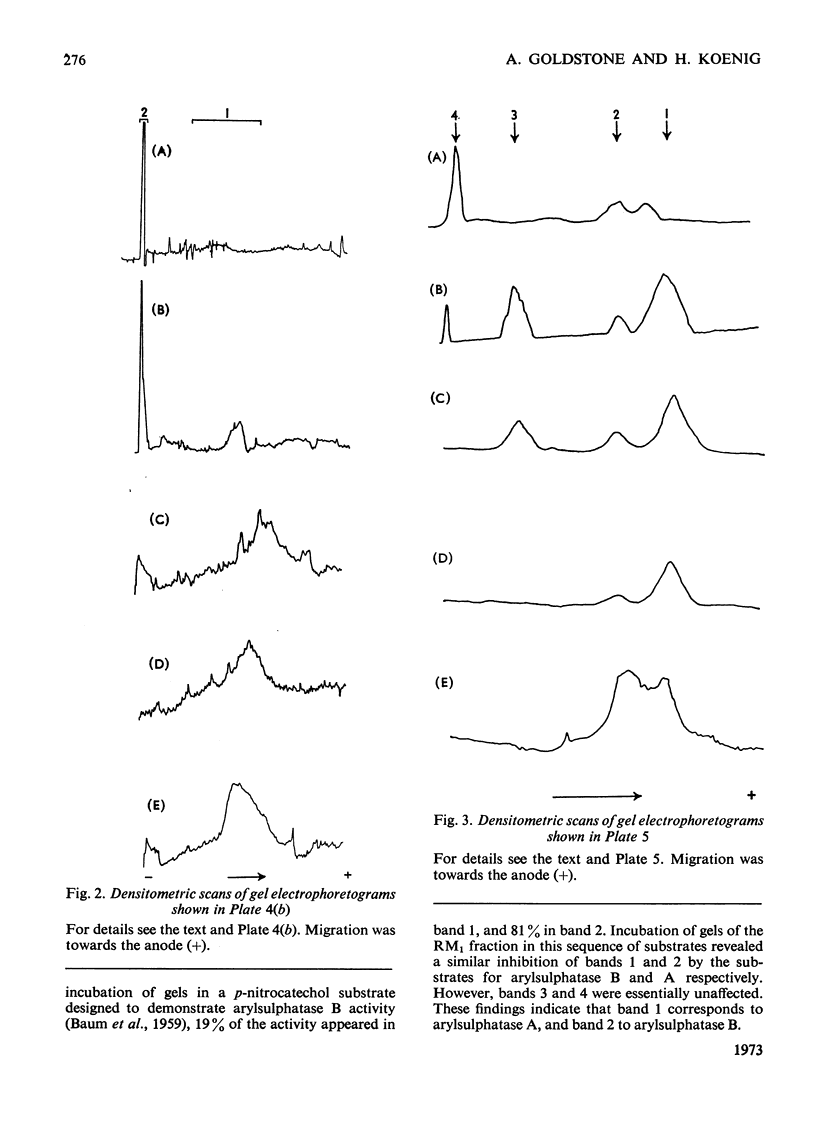
- BAUM H., DODGSON K. S., SPENCER B. The assay of arylsulphatases A and B in human urine. Clin Chim Acta. 1959 May;4(3):453–455. doi: 10.1016/0009-8981(59)90119-6. [DOI] [PubMed] [Google Scholar]
- Berlin J. D. The localization of acid mucopolysaccharides in the golgi complex of intestinal goblet cells. J Cell Biol. 1967 Mar;32(3):760–766. doi: 10.1083/jcb.32.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady R. O., Kolodny E. H. Disorders of ganglioside metabolism. Prog Med Genet. 1972;8:225–241. [PubMed] [Google Scholar]
- CARO L. G., PALADE G. E. PROTEIN SYNTHESIS, STORAGE, AND DISCHARGE IN THE PANCREATIC EXOCRINE CELL. AN AUTORADIOGRAPHIC STUDY. J Cell Biol. 1964 Mar;20:473–495. doi: 10.1083/jcb.20.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham R. D., Mooré D. J., Yunghans W. N. Isolation of a Golgi apparatus-rich fraction from rat liver. II. Enzymatic characterization and comparison with other cell fractions. J Cell Biol. 1970 Mar;44(3):492–500. doi: 10.1083/jcb.44.3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude A. Growth and differentiation of cytoplasmic membranes in the course of lipoprotein granule synthesis in the hepatic cell. I. Elaboration of elements of the Golgi complex. J Cell Biol. 1970 Dec;47(3):745–766. doi: 10.1083/jcb.47.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Fedorko M. E., Hirsch J. G. The in vitro differentiation of mononuclear phagocytes. V. The formation of macrophage lysosomes. J Exp Med. 1966 Apr 1;123(4):757–766. doi: 10.1084/jem.123.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Derechin M., Ostrowski W., Galka M., Barnard E. A. Acid phosphomonesterase of human prostate. Molecular weight, dissociation and chemical composition. Biochim Biophys Acta. 1971 Oct;250(1):143–154. doi: 10.1016/0005-2744(71)90128-8. [DOI] [PubMed] [Google Scholar]
- Dziembor E., Gryszkiewicz J., Ostrowski W. The role of neuraminic acid in the stability and enzymic activity of acid phosphatase of the human prostate gland. Experientia. 1970 Sep 26;26(9):947–948. doi: 10.1007/BF02114121. [DOI] [PubMed] [Google Scholar]
- Fedorko M. E., Hirsch J. G. Cytoplasmic granule formation in myelocytes. An electron microscope radioautographic study on the mechanism of formation of cytoplasmic granules in rabbit heterophilic myelocytes. J Cell Biol. 1966 May;29(2):307–316. doi: 10.1083/jcb.29.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Fleischer S., Ozawa H. Isolation and characterization of Golgi membranes from bovine liver. J Cell Biol. 1969 Oct;43(1):59–79. doi: 10.1083/jcb.43.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Fleischer S. Preparation and characterization of golgi membranes from rat liver. Biochim Biophys Acta. 1970 Dec 1;219(2):301–319. doi: 10.1016/0005-2736(70)90209-9. [DOI] [PubMed] [Google Scholar]
- Ganschow R. E., Bunker B. G. Genetic control of glucuronidase in mice. Biochem Genet. 1970 Feb;4(1):127–133. doi: 10.1007/BF00484025. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Koenig H. Lysosomal hydrolases as glycoproteins. Life Sci II. 1970 Dec 8;9(23):1341–1350. doi: 10.1016/0024-3205(70)90115-3. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Koenig H., Nayyar R., Hughes C., Lu C. Y. Isolation and characterization of a rough microsomal fraction from rat kidney that is enriched in lysosomal enzymes. Biochem J. 1973 Feb;132(2):259–266. doi: 10.1042/bj1320259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone A., Konecny P., Koenig H. Lysosomal hydrolases: Conversion of acidic to basic forms by neuraminidase. FEBS Lett. 1971 Feb 12;13(1):68–72. doi: 10.1016/0014-5793(71)80667-1. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Szabo E., Koenig H. Isolation and characterization of acidic lipoprotein in renal and hepatic lysosomes. Life Sci II. 1970 Jun 8;9(11):607–616. doi: 10.1016/0024-3205(70)90211-0. [DOI] [PubMed] [Google Scholar]
- HAYASHI M. DISTRIBUTION OF BETA-GLUCURONIDASE ACTIVITY IN RAT TISSUES EMPLOYING THE NAPHTHOL AS-BI GLUCURONIDE HEXAZONIUM PARAROSANILIN METHOD. J Histochem Cytochem. 1964 Sep;12:659–669. doi: 10.1177/12.9.659. [DOI] [PubMed] [Google Scholar]
- Holtzman E., Novikoff A. B., Villaverde H. Lysosomes and GERL in normal and chromatolytic neurons of the rat ganglion nodosum. J Cell Biol. 1967 May;33(2):419–435. doi: 10.1083/jcb.33.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide H., Fishman W. H. Dual localization of beta-glucuronidase and acid phosphatase in lysosomes and in microsomes. II. Membrane-associated enzymes. Histochemie. 1969;20(4):300–321. doi: 10.1007/BF00263748. [DOI] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. II. Transport to condensing vacuoles and zymogen granules. J Cell Biol. 1967 Aug;34(2):597–615. doi: 10.1083/jcb.34.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Hiroata I., Fishman W. H., Tsukamoto H. Intracellular transport of mouse kidney -glucuronidase induced by gonadotrophin. Biochem J. 1972 Apr;127(2):425–435. doi: 10.1042/bj1270425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Ide H., Shirahama T., Fishman W. H. Incorporation of [14C] glucosamine and [14C] leucine into mouse kidney beta-glucuronidase induced by gonadotrophin. Biochem J. 1970 Mar;117(1):161–167. doi: 10.1042/bj1170161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEHL E., JATZKEWITZ H. EVIDENCE FOR THE GENETIC BLOCK IN METACHROMATIC LEUCODYSTROPHY (ML). Biochem Biophys Res Commun. 1965 May 3;19:407–411. doi: 10.1016/0006-291x(65)90137-3. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H., WHALEY W. G. An observation on the functioning of the Golgi apparatus. J Cell Biol. 1963 Apr;17:222–225. doi: 10.1083/jcb.17.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Bennett B. D., Morré D. J., Gray M. E., Thistlethwaite W., LeQuire V. S. Lipoproteins associated with the Golgi apparatus isolated from epithelial cells of rat small intestine. Lab Invest. 1971 Nov;25(5):435–444. [PubMed] [Google Scholar]
- Mameli L., Potier M., Gianetto R. Difference in electrophoretic mobility between the lysosomal and the microsomal -glucuronidase of rat liver. Biochem Biophys Res Commun. 1972 Jan 31;46(2):560–563. doi: 10.1016/s0006-291x(72)80175-x. [DOI] [PubMed] [Google Scholar]
- Morre J., Merlin L. M., Keenan T. W. Localization of glycosyl transferase activities in a Golgi apparatus-rich fraction isolated from rat liver. Biochem Biophys Res Commun. 1969 Nov 20;37(5):813–819. doi: 10.1016/0006-291x(69)90964-4. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Hamilton R. L., Mollenhauer H. H., Mahley R. W., Cunningham W. P., Cheetham R. D., Lequire V. S. Isolation of a Golgi apparatus-rich fraction from rat liver. I. Method and morphology. J Cell Biol. 1970 Mar;44(3):484–491. doi: 10.1083/jcb.44.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVIKOFF A. B., ESSNER E., QUINTANA N. GOLGI APPARATUS AND LYSOSOMES. Fed Proc. 1964 Sep-Oct;23:1010–1022. [PubMed] [Google Scholar]
- Nichols B. A., Bainton D. F., Farquhar M. G. Differentiation of monocytes. Origin, nature, and fate of their azurophil granules. J Cell Biol. 1971 Aug;50(2):498–515. doi: 10.1083/jcb.50.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P. M., Novikoff A. B., Quintana N., Hauw J. J. Golgi apparatus, GERL, and lysosomes of neurons in rat dorsal root ganglia, studied by thick section and thin section cytochemistry. J Cell Biol. 1971 Sep;50(3):859–886. doi: 10.1083/jcb.50.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. 1969 Aug 15;165(3894):698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
- Okochi T., Masaki S., Aoki T., Ito F., Yamamura Y. Immunochemical studies on beta-glucuronidase isoenzyme. Med J Osaka Univ. 1968 Dec;19(2):147–155. [PubMed] [Google Scholar]
- Percy A. K., Brady R. O. Metachromatic leukodystrophy: diagnosis with samples of venous blood. Science. 1968 Aug 9;161(3841):594–595. doi: 10.1126/science.161.3841.594. [DOI] [PubMed] [Google Scholar]
- Price R. G., Dance N. The cellular distribution of some rat-kidney glycosidases. Biochem J. 1967 Nov;105(2):877–883. doi: 10.1042/bj1050877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley G. C., Pruyn M. L., Malt R. A. Glycoprotein synthesis by membraneound ribosomes and smooth membranes in kidney. Biochim Biophys Acta. 1969 Sep 17;190(1):154–160. doi: 10.1016/0005-2787(69)90164-6. [DOI] [PubMed] [Google Scholar]
- ROY A. B. Comparative studies on the liver sulphatases. Biochem J. 1958 Mar;68(3):519–528. doi: 10.1042/bj0680519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambourg A., Hernandez W., Leblond C. P. Detection of complex carbohydrates in the Golgi apparatus of rat cells. J Cell Biol. 1969 Feb;40(2):395–414. doi: 10.1083/jcb.40.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D., Price R. G., Dance N. Separation and properties of beta-galactosidase, beta-glucosidase, beta-glucuronidase and N-acetyl-beta-glucosaminidase from rat kidney. Biochem J. 1967 Feb;102(2):525–532. doi: 10.1042/bj1020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D., Stirling J. L. N-Acetyl-beta-glucosaminidases in human spleen. Biochem J. 1968 Apr;107(3):321–327. doi: 10.1042/bj1070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadahiro R., Takanashi S., Kawada M. Studies on the isozyme of beta-glucuronidase. J Biochem. 1965 Jul;58(1):104–106. doi: 10.1093/oxfordjournals.jbchem.a128156. [DOI] [PubMed] [Google Scholar]
- Sandhoff K. Variation of beta-N-acetylhexosaminidase-pattern in Tay-Sachs disease. FEBS Lett. 1969 Aug;4(4):351–354. doi: 10.1016/0014-5793(69)80274-7. [DOI] [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- Sloat B. F., Allen J. M. Soluble and membrane-associated forms of acid phosphatase associated with the lysosomal fraction of rat liver. Ann N Y Acad Sci. 1969 Oct 14;166(2):574–601. doi: 10.1111/j.1749-6632.1969.tb46421.x. [DOI] [PubMed] [Google Scholar]
- Smith J. K., Whitby L. G. The heterogeneity of prostatic acid phosphatase. Biochim Biophys Acta. 1968 Mar 25;151(3):607–618. doi: 10.1016/0005-2744(68)90007-7. [DOI] [PubMed] [Google Scholar]
- Smith R. E. Phosphohydrolases in cell organelles; electron microscopy. Ann N Y Acad Sci. 1969 Oct 14;166(2):525–564. doi: 10.1111/j.1749-6632.1969.tb46419.x. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y. Lipid synthesis, intracellular transport, storage, and secretion. I. Electron microscopic radioautographic study of liver after injection of tritiated palmitate or glycerol in fasted and ethanol-treated rats. J Cell Biol. 1967 May;33(2):319–339. doi: 10.1083/jcb.33.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lancker J. L., Lentz P. L. Study on the site of biosynthesis of beta-glucuronidase and its appearance in lysosome in normal and hypoxic rats. J Histochem Cytochem. 1970 Aug;18(8):529–541. doi: 10.1177/18.8.529. [DOI] [PubMed] [Google Scholar]
- WALKINSHAW C. H., MCCLURE H. M., VANLANCKER J. L. MOLECULAR MECHANISMS OF LIVER REGENERATION. III. THE EFFECT OF OSMOTIC SHOCK AND PH ON THE RELEASE OF PARTICULATE-BOUND ACID HYDROLASE IN NORMAL AND REGENERATING LIVER. Lab Invest. 1964 May;13:524–529. [PubMed] [Google Scholar]
- WALKINSHAW C. H., VANLANCKER J. L. MOLECULAR MECHANISMS OF LIVER REGENERATION. II. INTRACELLULAR DISTRIBUTION OF BIOCHEMICAL MARKERS IN NORMAL AND REGENERATING LIVER. Lab Invest. 1964 May;13:513–523. [PubMed] [Google Scholar]
- Wagner R. R., Cynkin M. A. Glycoprotein biosynthesis. Incorporation of glycosyl groups into endogenous acceptors in a Golgi apparatus-rich fraction of liver. J Biol Chem. 1971 Jan 10;246(1):143–151. [PubMed] [Google Scholar]