Abstract
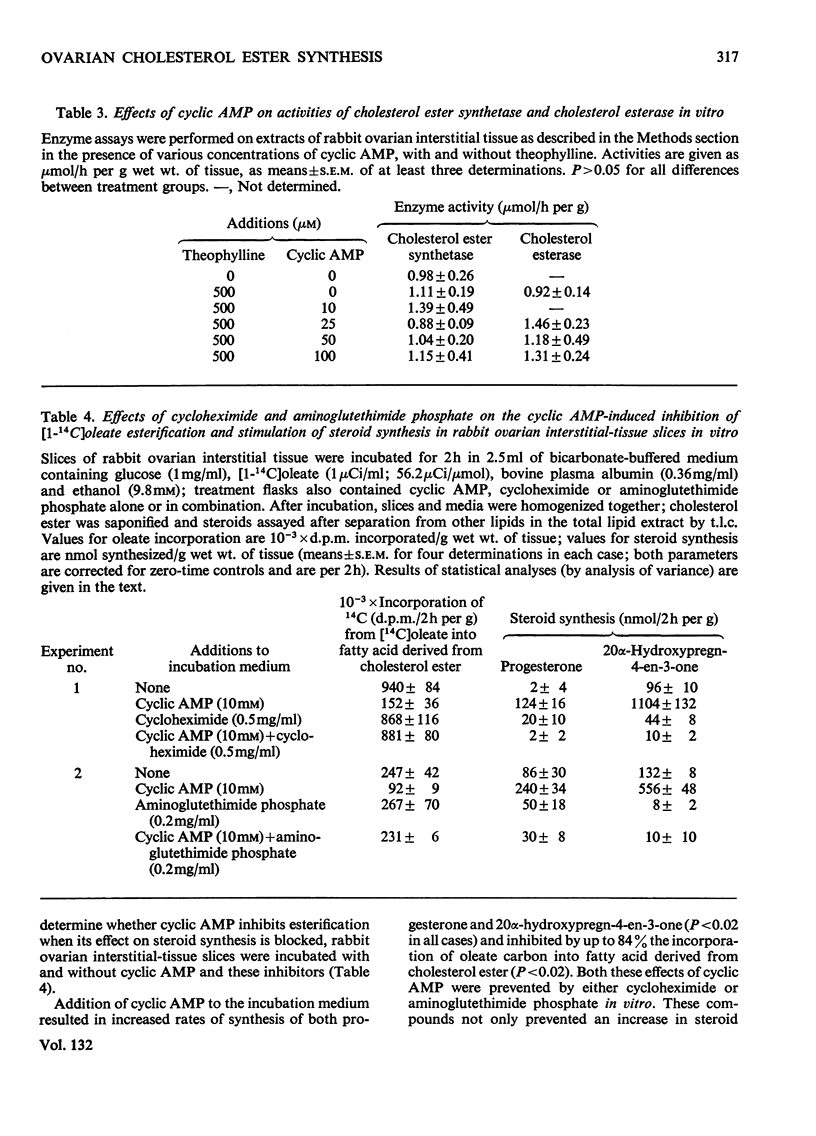
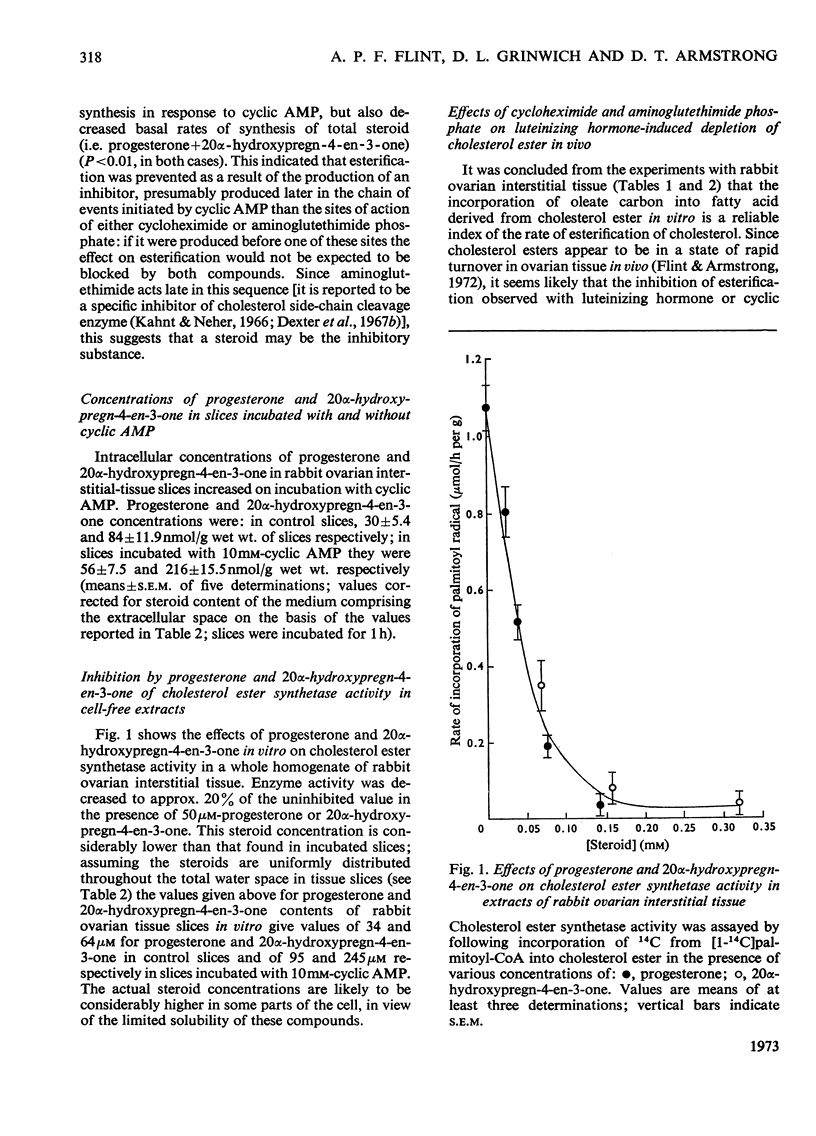
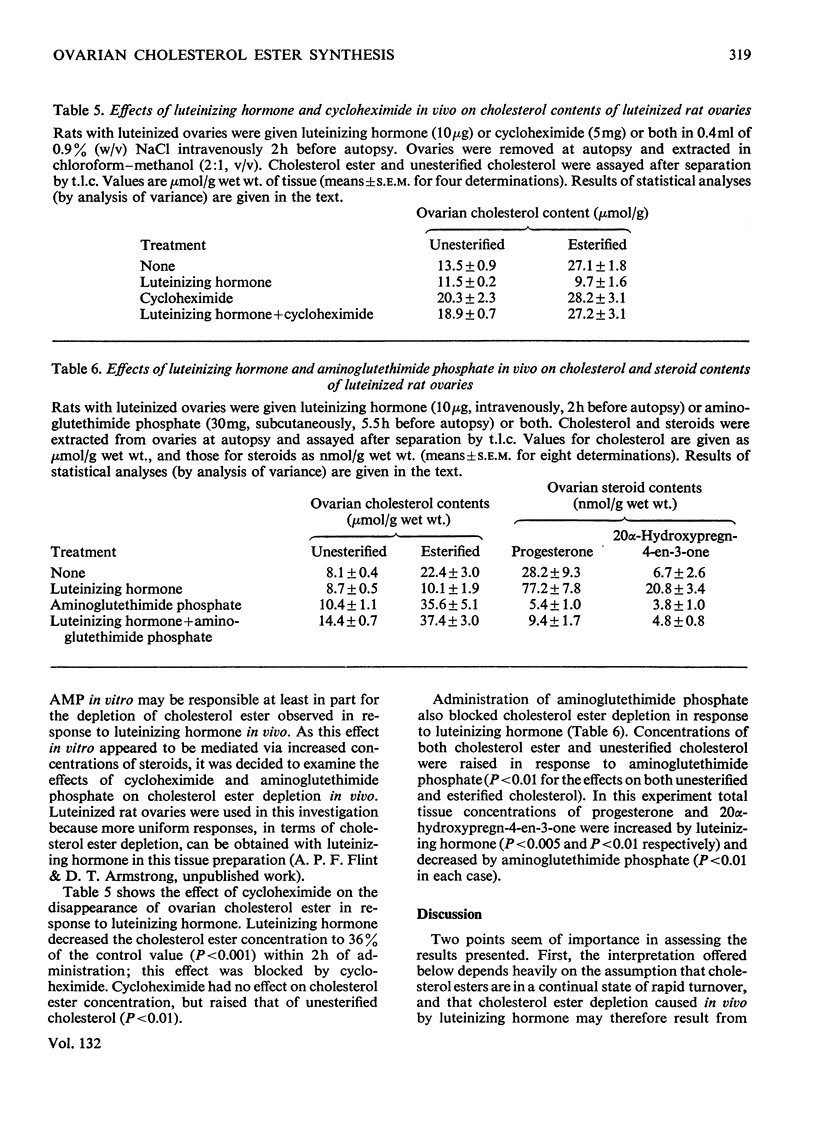
1. Experimental evidence is presented for a role of progesterone and 20α-hydroxypregn-4-en-3-one as inhibitors of cholesterol ester synthetase in the acute depletion of ovarian cholesterol ester after trophic stimulation. 2. Luteinizing hormone in vitro decreased by 84% the rate of esterification of cholesterol with added [14C]oleate by slices of rabbit ovarian interstitial tissue; this effect was mimicked by cyclic AMP (adenosine 3′:5′-cyclic monophosphate) in vitro, and occurred without large changes in precursor pool sizes or membrane permeability. 3. Cyclic AMP was shown to have no direct effect on cholesterol ester synthetase or cholesterol esterase in cell-free extracts of rabbit ovarian interstitial tissue, but decreased the activity of cholesterol ester synthetase (not that of cholesterol esterase) in extracts prepared from slices previously incubated with it. 4. The inhibitory effect of cyclic AMP on esterification of cholesterol with added [14C]-oleate was prevented by both cycloheximide and aminoglutethimide phosphate (which also inhibited steroid synthesis in response to cyclic AMP). 5. Cyclic AMP raised the intracellular concentrations of progesterone and 20α-hydroxypregn-4-en-3-one in incubated slices by factors of 2.8 and 3.9 respectively. 6. Cycloheximide and aminoglutethimide phosphate administered in vivo blocked cholesterol ester depletion in response to luteinizing hormone in rats; in these ovaries cycloheximide and aminoglutethimide phosphate decreased the concentrations of progesterone and 20α-hydroxypregn-4-en-3-one and luteinizing hormone raised them. 7. Progesterone and 20α-hydroxypregn-4-en-3-one added to cell-free extracts of rabbit ovarian interstitial tissue in vitro (at concentrations comparable with those found in incubated slices) inhibited cholesterol ester synthetase by up to 85%. 8. The results are discussed with reference to the acute control of cholesterol ester concentrations in the ovary and adrenal cortex.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. T. Gonadotropins, ovarian metabolism, and steroid biosynthesis. Recent Prog Horm Res. 1968;24:255–319. doi: 10.1016/b978-1-4831-9827-9.50012-5. [DOI] [PubMed] [Google Scholar]
- Behrman H. R., Amstrong D. T. Cholesterol esterase stimulation by luteinizing hormone in luteinized rat ovaries. Endocrinology. 1969 Sep;85(3):474–480. doi: 10.1210/endo-85-3-474. [DOI] [PubMed] [Google Scholar]
- Behrman H. R., Orczyk G. P., Macdonald G. J., Greep R. O. Prolactin induction of enzymes controlling luteal cholesterol ester turnover. Endocrinology. 1970 Dec;87(6):1251–1256. doi: 10.1210/endo-87-6-1251. [DOI] [PubMed] [Google Scholar]
- Berhman H. R., Armstrong D. T., Greep R. O. Studies on the rapid cholesterol-depleting and steroidogenic actions of luteinizing hormone in the rat ovary: effects of aminoglutethimide phosphate. Can J Biochem. 1970 Aug;48(8):881–884. doi: 10.1139/o70-138. [DOI] [PubMed] [Google Scholar]
- CLAESSON L., HILLARP N. A., HOGBERG B. Lipid changes in the interstitial gland of the rabbit ovary at oestrogen formation. Acta Physiol Scand. 1953 Nov 17;29(4):329–339. doi: 10.1111/j.1748-1716.1953.tb01028.x. [DOI] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Davis W. W., Garren L. D. On the mechanism of action of adrenocorticotropic hormone. The inhibitory site of cycloheximide in the pathway of steroid biosynthesis. J Biol Chem. 1968 Oct 10;243(19):5153–5157. [PubMed] [Google Scholar]
- Denton R. M., Halperin M. L. The control of fatty acid and triglyceride synthesis in rat epididymal adipose tissue. Roles of coenzyme A derivatives, citrate and L-glycerol 3-phosphate. Biochem J. 1968 Nov;110(1):27–38. doi: 10.1042/bj1100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter R. N., Fishman L. M., Ney R. L., Liddle G. W. An effect of adrenocorticotrophic hormone on adrenal cholesterol accumulation. Endocrinology. 1967 Nov;81(5):1185–1187. doi: 10.1210/endo-81-5-1185. [DOI] [PubMed] [Google Scholar]
- Dexter R. N., Fishman L. M., Ney R. L., Liddle G. W. Inhibition of adrenal corticosteroid synthesis by aminoglutethimide: studies of the mechanism of action. J Clin Endocrinol Metab. 1967 Apr;27(4):473–480. doi: 10.1210/jcem-27-4-473. [DOI] [PubMed] [Google Scholar]
- Dorrington J. H., Baggett B. Adenyl cyclase activity in the rabbit ovary. Endocrinology. 1969 May;84(5):989–996. doi: 10.1210/endo-84-5-989. [DOI] [PubMed] [Google Scholar]
- FARESE R. V. INHIBITION OF THE STEROIDOGENIC EFFECT OF ACTH AND INCORPORATION OF AMINO ACID INTO RAT ADRENAL PROTEIN IN VITRO BY CHLORAMPHENICOL. Biochim Biophys Acta. 1964 Aug 12;87:699–701. doi: 10.1016/0926-6550(64)90292-0. [DOI] [PubMed] [Google Scholar]
- FERGUSON J. J., Jr PROTEIN SYNTHESIS AND ADRENOCORTICOTROPIN RESPONSIVENESS. J Biol Chem. 1963 Aug;238:2754–2759. [PubMed] [Google Scholar]
- Flint A. P., Armstrong D. T. Activities of enzymes responsible for steroid biosynthesis and cholesterol ester metabolism in rabbit ovarian interstitial tissue and corpora lutea. A comparison of enzyme activities with flow rates. Biochem J. 1973 Feb;132(2):301–311. doi: 10.1042/bj1320301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A. P., Armstrong D. T. The compartmentation of non-esterified and esterified cholesterol in the superovulated rat ovary. Biochem J. 1971 Jun;123(2):143–152. doi: 10.1042/bj1230143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Shepherd D., Yates D. W. Steady-state concentrations of coenzyme A, acetyl-coenzyme A and long-chain fatty acyl-coenzyme A in rat-liver mitochondria oxidizing palmitate. Biochem J. 1965 Nov;97(2):587–594. doi: 10.1042/bj0970587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Padnos D. Translational control of protein synthesis and the control of steroidogenesis in the rabbit ovary. Arch Biochem Biophys. 1966 Jan;113(1):100–106. doi: 10.1016/0003-9861(66)90161-5. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith D. G., Butcher R. W., Ney R. L., Sutherland E. W. Adenosine 3',5'-monophosphate as the intracellular mediator of the action of adrenocorticotropic hormone on the adrenal cortex. J Biol Chem. 1967 Dec 10;242(23):5535–5541. [PubMed] [Google Scholar]
- HAYNES R. C., Jr The activation of adrenal phosphorylase by the adrenocorticotropic hormone. J Biol Chem. 1958 Nov;233(5):1220–1222. [PubMed] [Google Scholar]
- Johnston G. G., Krisle J. R., Troop R. C. Inhibition of adrenal steroidogenesis by glutethimide. Proc Soc Exp Biol Med. 1968 Oct;129(1):20–23. doi: 10.3181/00379727-129-33239. [DOI] [PubMed] [Google Scholar]
- MUKHERJEE S., KUNITAKE G., ALFINSLATER R. B. The esterification of cholesterol with palmitic acid by rat liver homogenates. J Biol Chem. 1958 Jan;230(1):91–96. [PubMed] [Google Scholar]
- Marsh J. M., Butcher R. W., Savard K., Sutherland E. W. The stimulatory effect of luteinizing hormone on adenosine 3',5'-monophosphate accumulation in corpus luteum slices. J Biol Chem. 1966 Nov 25;241(22):5436–5440. [PubMed] [Google Scholar]
- RANDLE P. J., SMITH G. H. Regulation of glucose uptake by muscle. 2. The effects of insulin, anaerobiosis and cell poisons on the penetration of isolated rat diaphragm by sugars. Biochem J. 1958 Nov;70(3):501–508. doi: 10.1042/bj0700501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANADI D. R., LITTLEFIELD J. W., BOCK R. M. Studies on alpha-ketoglutaric oxidase. II. Purification and properties. J Biol Chem. 1952 May;197(2):851–862. [PubMed] [Google Scholar]
- SAVARD K., MARSH J. M., RICE B. F. GONADOTROPINS AND OVARIAN STEROIDOGENESIS. Recent Prog Horm Res. 1965;21:285–365. [PubMed] [Google Scholar]
- Wilks J. W., Fuller G. B., Hansel W. The role of cholesterol as a progestin precursor in rat, rabbit, and bovine luteal tissue. Endocrinology. 1970 Sep;87(3):581–587. doi: 10.1210/endo-87-3-581. [DOI] [PubMed] [Google Scholar]
- Wilroy R. S., Jr, Camacho A. M., Trouy R. L., Hagen A. A. Inhibition of adrenal cortical secretion by amino-glutethimide in dogs. Endocrinology. 1968 Jul;83(1):56–60. doi: 10.1210/endo-83-1-56. [DOI] [PubMed] [Google Scholar]
- Zarrow M. X., Clark J. H. Gonadotropin regulation of ovarian cholesterol levels in the rat. Endocrinology. 1969 Feb;84(2):340–346. doi: 10.1210/endo-84-2-340. [DOI] [PubMed] [Google Scholar]


