Abstract
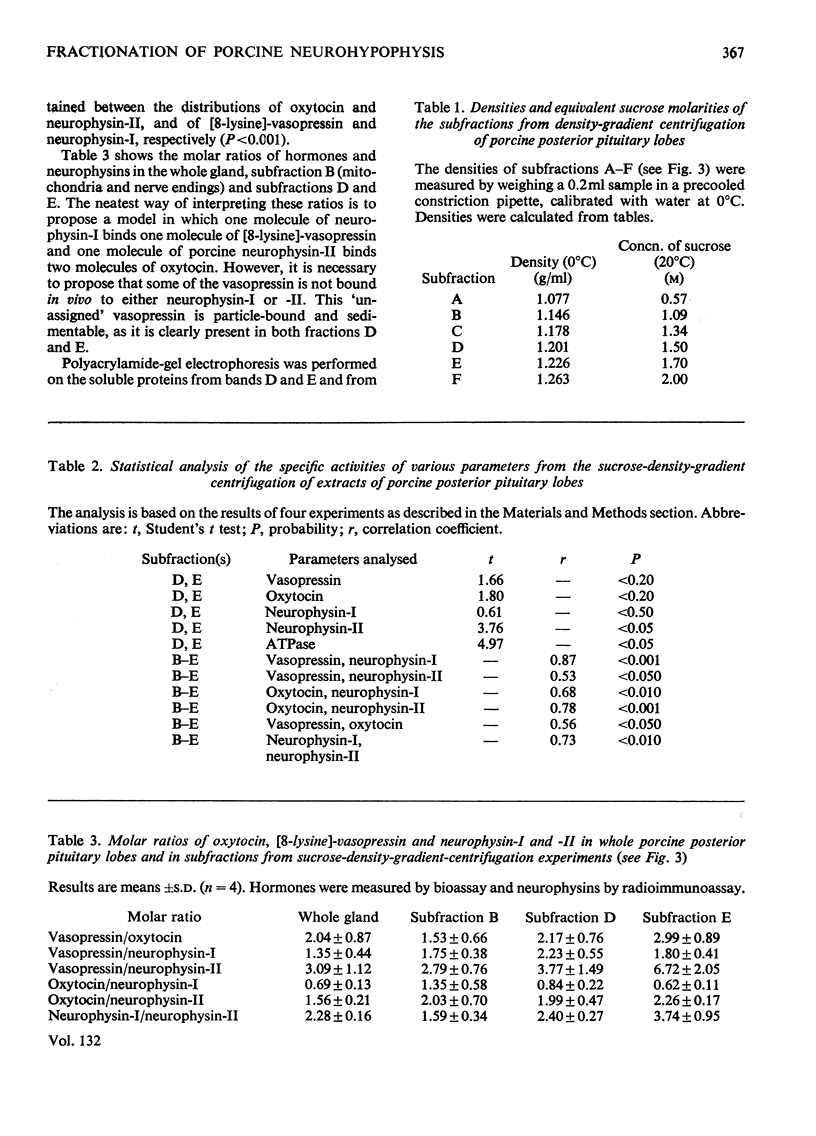
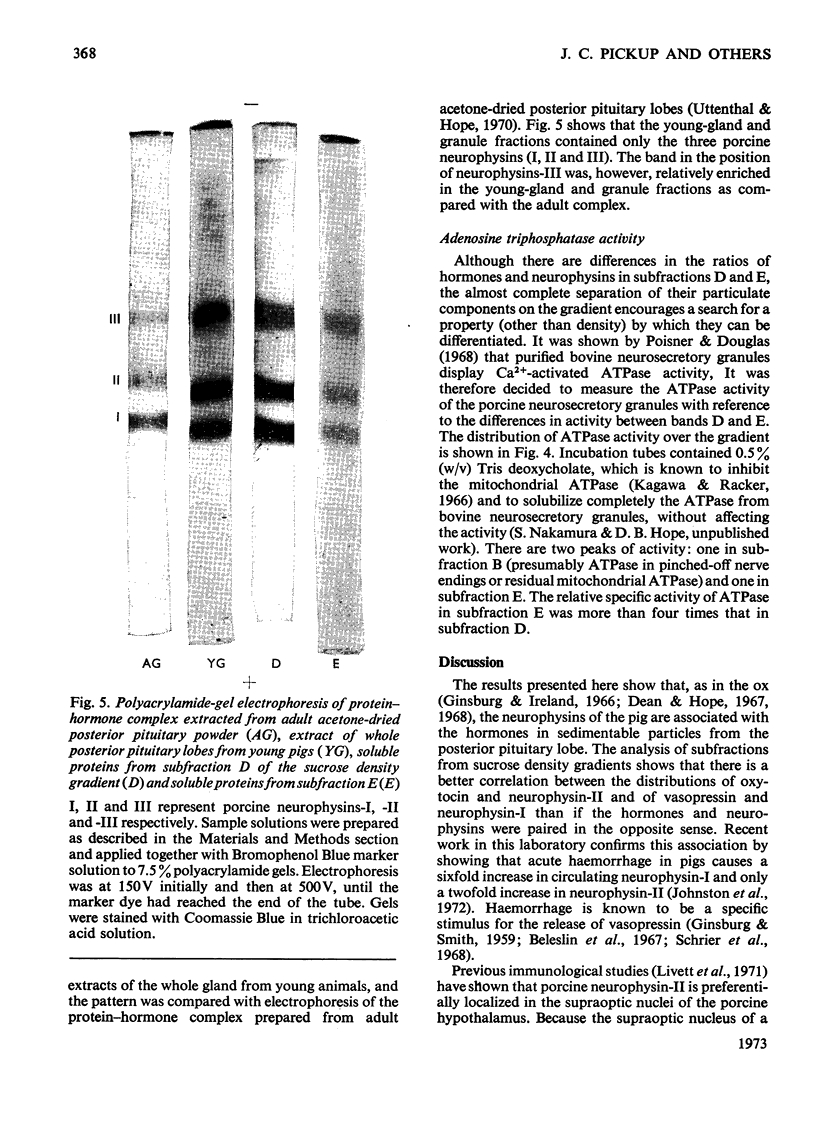
Posterior pituitary lobes from young pigs were fractionated by differential and sucrose-density-gradient centrifugation. The distributions of oxytocin and [8-lysine]-vasopressin were measured by bioassay and the distributions of neurophysin-I and -II by radioimmunoassays specific for each of these two proteins. Most of the hormone and neurophysin applied to the density gradient was localized in particles with the density expected of neurosecretory granules. However, the neurosecretory granules were separated into two bands (D and E). A close statistical correlation between the distributions of [8-lysine]-vasopressin and neurophysin-I, and of oxytocin and neurophysin-II on the gradients, suggested that in vivo porcine neurophysin-I binds [8-lysine]-vasopressin within one population of granules and porcine neurophysin-II binds oxytocin within another type of granule. However, there was no significant separation of oxytocin and vasopressin in fractions D and E. The molar ratios of hormones and neurophysins indicated that there was insufficient of either neurophysin to bind the [8-lysine]-vasopressin in the granule fractions or in the whole gland. Polyacrylamide-gel electrophoresis showed that only bands corresponding in mobility to porcine neurophysins-I, -II and -III were present in large amounts in the whole gland and in the granule fractions. The component with the mobility of neurophysin-III was, however, relatively enriched in whole young glands and granule fractions compared with adult gland extracts. It is suggested that the vasopressin that cannot be assigned to neurophysin-I may occur in (a) vesicles containing vasopressin but no neurophysin, (b) vesicles containing vasopressin and a protein that cannot be quantified by the radioimmunoassays used, such as porcine neurophysin-III, or (c) normal vasopressin–neurophysin granules which have accumulated extra vasopressin. Band E of the gradient was rich in adenosine triphosphatase activity, whereas band D possessed very little of this enzyme.
Full text
PDF


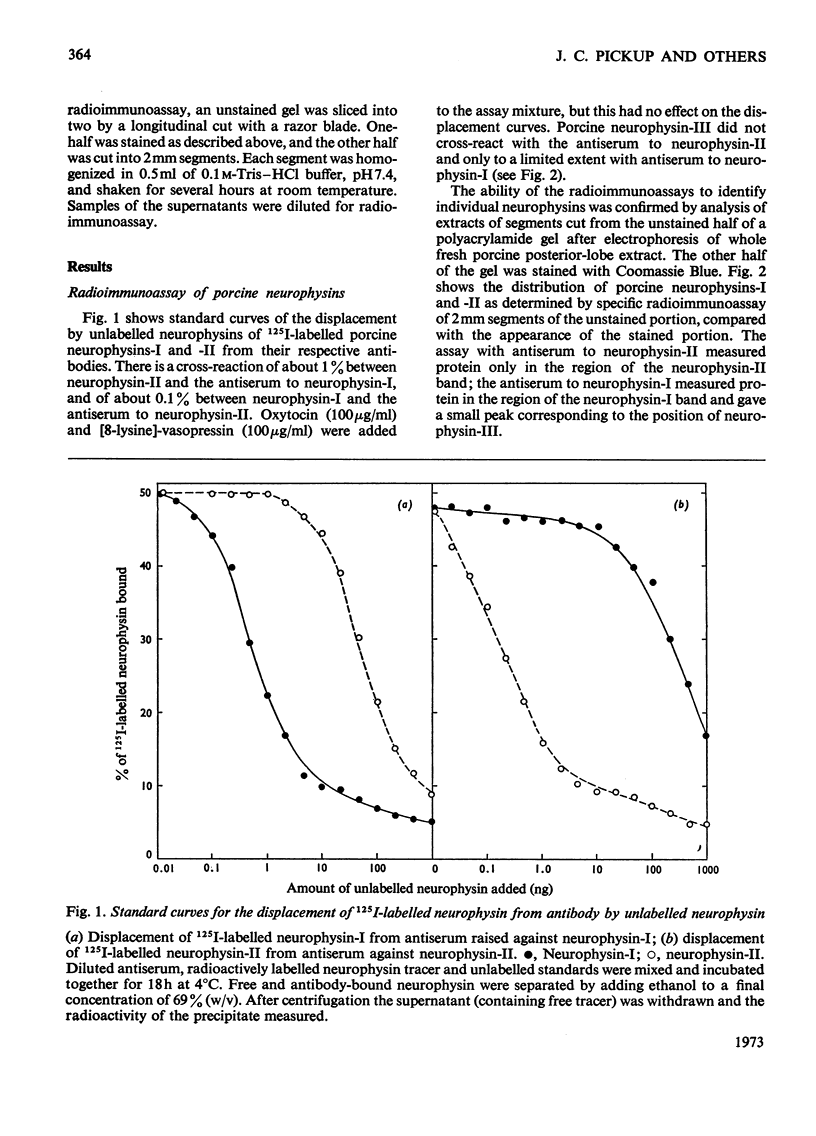
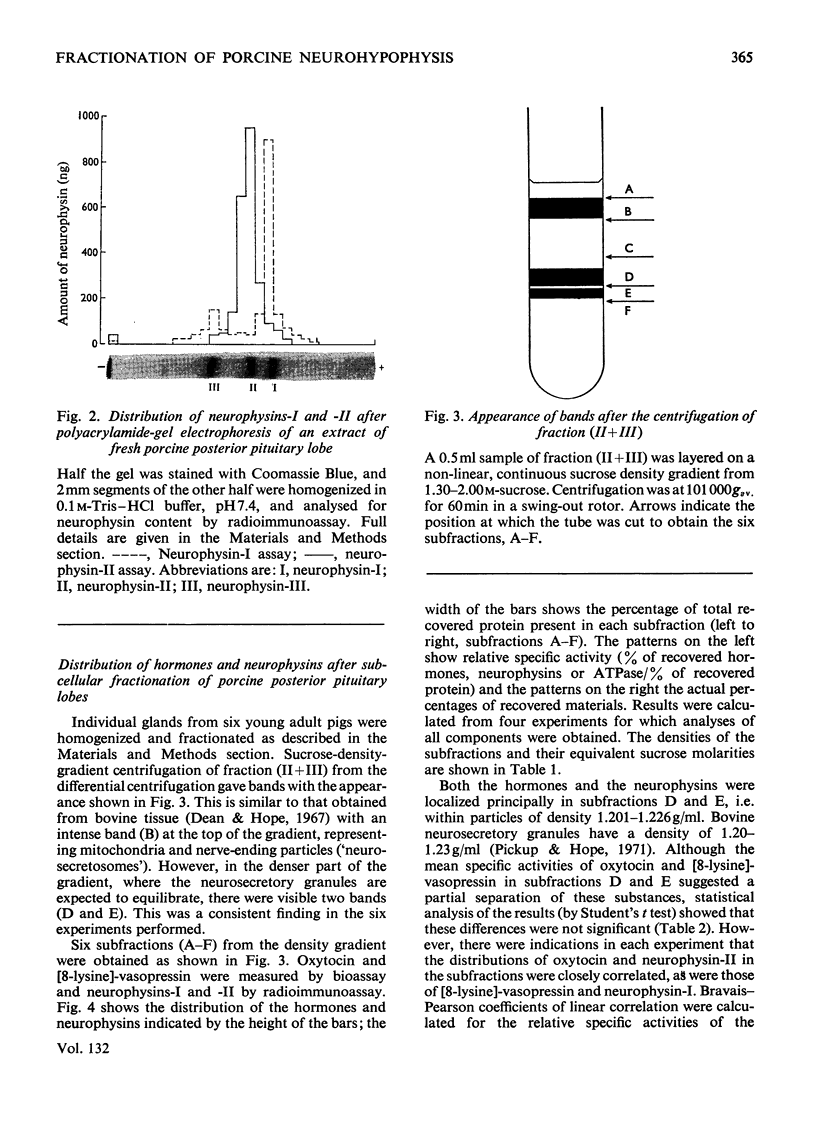
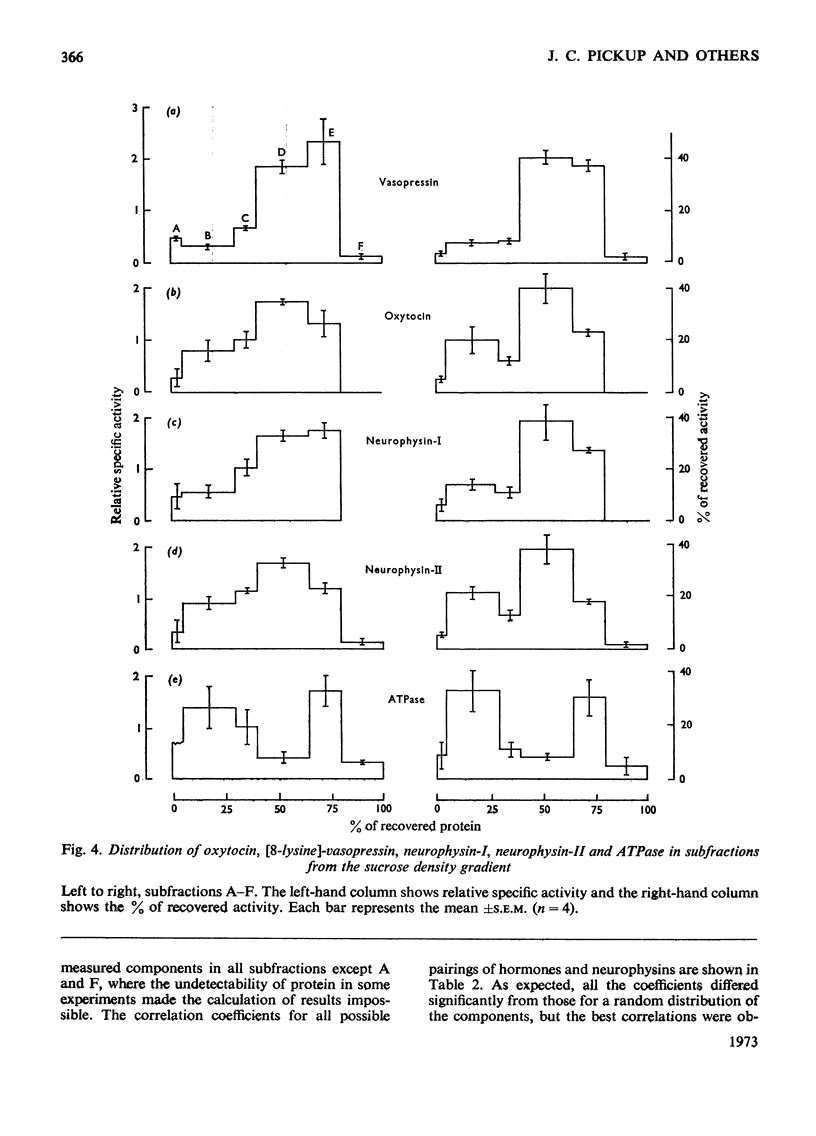



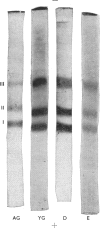
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AROSKAR J. P., CHAN W. Y., STOUFFER J. E., SCHNEIDER C. H., MURTI V. V., DUVIGNEAUD V. RENAL EXCRETION AND TISSUE DISTRIBUTION OF RADIOACTIVITY AFTER ADMINISTRATION OF TRITIUM-LABELED OXYTOCIN TO RATS. Endocrinology. 1964 Feb;74:226–232. doi: 10.1210/endo-74-2-226. [DOI] [PubMed] [Google Scholar]
- Aulsebrook L. H., Holland R. C. Central regulation of oxytocin release with and without vasopressin release. Am J Physiol. 1969 Apr;216(4):818–829. doi: 10.1152/ajplegacy.1969.216.4.818. [DOI] [PubMed] [Google Scholar]
- BANGHAM D. R., MUSSETT M. V. Third international standard for posterior pituitary; re-named third international standard for oxytocic, vasopressor and antidiuretic substances in 1956. Bull World Health Organ. 1958;19(2):325–340. [PMC free article] [PubMed] [Google Scholar]
- BARER R., HELLER H., LEDERIS K. THE ISOLATION, IDENTIFICATION AND PROPERTIES OF THE HORMONAL GRANULES OF THE NEUROHYPOPHYSIS. Proc R Soc Lond B Biol Sci. 1963 Oct 22;158:388–416. doi: 10.1098/rspb.1963.0054. [DOI] [PubMed] [Google Scholar]
- Bindler E., Labella F. S., Sanwal M. Isolated nerve endings (neurosecretosomes) from the posterior pituitary. Partial separation of vasopressin and oxytocin and the isolation of microvesicles. J Cell Biol. 1967 Jul;34(1):185–205. doi: 10.1083/jcb.34.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J., Errington M. L. The hypothalamic neurosecretory pathways for the release of oxytocin and vasopressin in the cat. J Physiol. 1971 Aug;217(1):111–131. doi: 10.1113/jphysiol.1971.sp009562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J., Haldar J. Blood levels of oxytocin and vasopressin during suckling in the rabbit and the problem of their independent release. J Physiol. 1970 Mar;206(3):711–722. doi: 10.1113/jphysiol.1970.sp009039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow E., Abrash L. The binding of oxytocin and oxytocin analogues by purified bovine neurophysins. Proc Natl Acad Sci U S A. 1966 Aug;56(2):640–646. doi: 10.1073/pnas.56.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow E., Walter R. Binding properties of bovine neurophysins I and II: an equilibrium dialysis study. Mol Pharmacol. 1972 Jan;8(1):75–81. [PubMed] [Google Scholar]
- Burford G. D., Jones C. W., Pickering B. T. Tentative identification of a vasopressin-neurophysin and an oxytocin-neurophysin in the rat. Biochem J. 1971 Oct;124(4):809–813. doi: 10.1042/bj1240809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE J. T. SIMPLIFIED "DISC" (POLYACRYLAMIDE GEL) ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:428–436. doi: 10.1111/j.1749-6632.1964.tb14214.x. [DOI] [PubMed] [Google Scholar]
- Campbell D. J., Holmes R. L. Further studies on the neurohypophysis of the hedgehog (Erinaceus europaeus). Z Zellforsch Mikrosk Anat. 1966;75(1):35–46. doi: 10.1007/BF00407142. [DOI] [PubMed] [Google Scholar]
- Cheng K. W., Friesen H. G. Physiological factors regulating secretion of neurophysin. Metabolism. 1970 Oct;19(10):876–890. doi: 10.1016/0026-0495(70)90085-5. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Clark B. J., Silva MR Jr E. An afferent pathway for the selective release of vasopressin in response to carotid occlusion and haemorrhage in the cat. J Physiol. 1967 Aug;191(3):529–542. doi: 10.1113/jphysiol.1967.sp008266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEKANSKI J. The quantitative assay of vasopressin. Br J Pharmacol Chemother. 1952 Dec;7(4):567–572. doi: 10.1111/j.1476-5381.1952.tb00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKER S. E., TYLER C. Estimation of the antidiuretic, vasopressor and oxytocic hormones in the pituitary gland of dogs and puppies. J Physiol. 1953 Apr 28;120(1-2):141–145. doi: 10.1113/jphysiol.1953.sp004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. R., Hope D. B. The isolation of neurophysin-I and-II from bovine pituitary neurosecretory granules separated on a large scale from other subcellular organelles. Demonstration of slow equilibration of neurosecretory granules during centrifugation in a sucrose density gradient. Biochem J. 1968 Jan;106(2):565–573. doi: 10.1042/bj1060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. R., Hope D. B. The isolation of purified neurosecretory granules from bovine pituitary posterior lobes. Comparison of granule protein constituents with those of neurophysin. Biochem J. 1967 Sep;104(3):1082–1088. doi: 10.1042/bj1041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker S. E. Release of vasopressin and oxytocin from isolated pituitary glands of adult and new-born rats. J Physiol. 1966 Jul;185(2):429–444. doi: 10.1113/jphysiol.1966.sp007994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGGSTEIN M., KREUTZ F. H. Vergleichende Untersuchungen zur quantitativen Eiweissbestimmung im Liquor und eiweissarmen Lösungen. Klin Wochenschr. 1955 Oct 1;33(37-38):879–884. doi: 10.1007/BF01473099. [DOI] [PubMed] [Google Scholar]
- Edwards B. A. The uptake of tritiated lysine vasopressin by rat and pig pituitary neural lobes in vitro. J Endocrinol. 1971 Aug;50(4):669–677. doi: 10.1677/joe.0.0500669. [DOI] [PubMed] [Google Scholar]
- Edwards B. A. Uptake of tritiated oxytocin into the neurohypophysis of the rat. J Endocrinol. 1971 Nov;51(3):607–608. doi: 10.1677/joe.0.0510607. [DOI] [PubMed] [Google Scholar]
- Fawcett C. P., Powell A. E., Sachs H. Biosynthesis and release of neurophysin. Endocrinology. 1968 Dec;83(6):1299–1310. doi: 10.1210/endo-83-6-1299. [DOI] [PubMed] [Google Scholar]
- Furth A. J., Hope D. B. Studies on the chemical modification of the tyrosine residue in bovine neurophysin-II. Biochem J. 1970 Feb;116(4):545–553. doi: 10.1042/bj1160545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAITAN E., COBO E., MIZRACHI M. EVIDENCE FOR THE DIFFERENTIAL SECRETION OF OXYTOCIN AND VASOPRESSIN IN MAN. J Clin Invest. 1964 Dec;43:2310–2322. doi: 10.1172/JCI105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBURG M., SMITH M. W. The fate of oxytocin in male and female rats. Br J Pharmacol Chemother. 1959 Sep;14:327–333. doi: 10.1111/j.1476-5381.1959.tb00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg M., Ireland M. The role of neurophysin in the transport and release of neurohypophysical hormones. J Endocrinol. 1966 Jul;35(3):289–298. doi: 10.1677/joe.0.0350289. [DOI] [PubMed] [Google Scholar]
- Ginsburg M. Molecular aspects of neurohypophysial hormone release. Proc R Soc Lond B Biol Sci. 1968 May 14;170(1018):27–36. doi: 10.1098/rspb.1968.0021. [DOI] [PubMed] [Google Scholar]
- HELLER H., LEDERIS K. Maturation of the hypothalamoneurohypophysial system. J Physiol. 1959 Sep 2;147:299–314. doi: 10.1113/jphysiol.1959.sp006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Haldar J. Independent release of oxytocin and vasopressin during parturition in the rabbit. J Physiol. 1970 Mar;206(3):723–730. doi: 10.1113/jphysiol.1970.sp009040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Hope D. B. The isolation of the native hormone-binding proteins from bovine pituitary posterior lobes. Crystallization of neurophysin-I and-II as complexes with [8-arginine]-vasopressin. Biochem J. 1968 Jan;106(2):557–564. doi: 10.1042/bj1060557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 8. Properties of a factor conferring oligomycin sensitivity on mitochondrial adenosine triphosphatase. J Biol Chem. 1966 May 25;241(10):2461–2466. [PubMed] [Google Scholar]
- LABELLA F. S., BEAULIEU G., REIFFENSTEIN R. J. Evidence for the existence of separate vasopressin and oxytocin containing granules in the neurohypophysis. Nature. 1962 Jan 13;193:173–174. doi: 10.1038/193173a0. [DOI] [PubMed] [Google Scholar]
- LEDERIS K. FINE STRUCTURE AND HORMONE CONTENT OF THE HYPOTHALAMO-NEUROHYPOPHYSIAL SYSTEM OF THE RAINBOW TROUT (SALMO IRIDEUS) EXPOSED TO SEA WATER. Gen Comp Endocrinol. 1964 Dec;4:638–661. doi: 10.1016/0016-6480(64)90074-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NIBBELINK D. W. Paraventricular nuclei, neurohypophysis, and parturition. Am J Physiol. 1961 Jun;200:1229–1232. doi: 10.1152/ajplegacy.1961.200.6.1229. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Dreifuss J. J., Legros J. J. A correlation of release of polypeptide hormones and of immunoreactive neurophysin from isolated rat neurohypophyses. Experientia. 1971;27(11):1344–1345. doi: 10.1007/BF02136730. [DOI] [PubMed] [Google Scholar]
- Norström A., Sjöstrand J. Effect of haemorrhage on the rapid axonal transport of neurohypophysial proteins of the rat. J Neurochem. 1971 Nov;18(11):2017–2026. doi: 10.1111/j.1471-4159.1971.tb05061.x. [DOI] [PubMed] [Google Scholar]
- Pickup J. C., Hope D. B. Protease and ribonuclease activities in bovine pituitary lobes. Biochem J. 1971 Jun;123(2):153–162. doi: 10.1042/bj1230153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliska V., Thorn N. A., Vilhardt H. In vitro uptake and breakdown of tritiated lysine-vasopressin by bovine neurohypophyseal and cortical tissue. Acta Endocrinol (Copenh) 1971 May;67(1):12–22. doi: 10.1530/acta.0.0670012. [DOI] [PubMed] [Google Scholar]
- Poisner A. M., Douglas W. W. A possible mechanism of release of posterior pituitary hormones involving adenosine triphosphate and an adenosine triphosphatase in the neurosecretory granules. Mol Pharmacol. 1968 Sep;4(5):531–540. [PubMed] [Google Scholar]
- Rauch R., Hollenberg M. D., Hope D. B. Isolation of a third bovine neurophysin. Biochem J. 1969 Nov;115(3):473–479. doi: 10.1042/bj1150473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs H., Fawcett P., Takabatake Y., Portanova R. Biosynthesis and release of vasopressin and neurophysin. Recent Prog Horm Res. 1969;25:447–491. doi: 10.1016/b978-0-12-571125-8.50013-2. [DOI] [PubMed] [Google Scholar]
- Sattin A., Rall T. W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3', 5'-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970 Jan;6(1):13–23. [PubMed] [Google Scholar]
- Schrier R. W., Verroust P. J., Jones J. J., Fabian M., Lee J., De Wardener H. E. Effect of isotonic saline infusion and acute haemorrhage on plasma oxytocin and vasopressin concentrations in dogs. Clin Sci. 1968 Dec;35(3):433–443. [PubMed] [Google Scholar]
- Thorn N. A. In vitro studies of the release mechanism for vasopressin in rats. Acta Endocrinol (Copenh) 1966 Dec;53(4):644–654. doi: 10.1530/acta.0.0530644. [DOI] [PubMed] [Google Scholar]
- Tindal J. S., Knaggs G. S., Turvey A. Preferential release of oxytocin from the neurohypophysis after electrical stimulation of the afferent path of the milk-ejection reflex in the brain of the guinea-pig. J Endocrinol. 1968 Feb;40(2):205–214. doi: 10.1677/joe.0.0400205. [DOI] [PubMed] [Google Scholar]
- Uttenthal L. O., Hope D. B. Neurophysins and posterior pituitary hormones in the suiformes. Proc R Soc Lond B Biol Sci. 1972 Jul 25;182(1066):73–87. doi: 10.1098/rspb.1972.0067. [DOI] [PubMed] [Google Scholar]
- Uttenthal L. O., Hope D. B. The isolation of three neurophysins from porcine posterior pituitary lobes. Biochem J. 1970 Mar;116(5):899–909. doi: 10.1042/bj1160899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttenthal L. O., Livett B. G., Hope D. B. Release of neurophysin together with vasopressin by a Ca 2 dependent mechanism. Philos Trans R Soc Lond B Biol Sci. 1971 Jun 17;261(839):379–380. doi: 10.1098/rstb.1971.0068. [DOI] [PubMed] [Google Scholar]
- Uttenthal L. O., Livett B. G., Hope D. B. Release of neurophysin together with vasopressin by a Ca 2 dependent mechanism. Philos Trans R Soc Lond B Biol Sci. 1971 Jun 17;261(839):379–380. doi: 10.1098/rstb.1971.0068. [DOI] [PubMed] [Google Scholar]
- Vilhardt H., Tondevold E. Studies on isolated secretory granules from bovine neurohypophyses. Distribution of labelled granules on a density gradient. Release of tritiated lysine-vasopressin and endogenous arginine-vasopressin during stimulation procedures. Acta Endocrinol (Copenh) 1972 Aug;70(4):625–635. [PubMed] [Google Scholar]
- Vilhardt H. Uptake of tritiated lysine-vasopressin by isolated secretory granules from bovine neurohypophyses. Acta Endocrinol (Copenh) 1971 Nov;68(3):417–424. doi: 10.1530/acta.0.0680417. [DOI] [PubMed] [Google Scholar]
- Vizsolyi E., Perks A. M. New neurohypophysial principle in foetal mammals. Nature. 1969 Sep 13;223(5211):1169–1171. doi: 10.1038/2231169a0. [DOI] [PubMed] [Google Scholar]
- Willumsen N. B., Bie P. Tissue to plasma ratios of radioactivity in the rat hypothalamo-hypophyseal system after intraveous injection of 3H-lysine-vasopressin and 3H-mannitol. Acta Endocrinol (Copenh) 1969 Mar;60(3):389–400. doi: 10.1530/acta.0.0600389. [DOI] [PubMed] [Google Scholar]
- Wuu T. C., Saffran M. Isolation and characterization of a hormone-binding polypeptide from pig posterior pituitary powder. J Biol Chem. 1969 Jan 25;244(2):482–490. [PubMed] [Google Scholar]
- Zambrano D., De Robertis E. Ultrastructural changes of the neurohypophysis of the rat after castration. Z Zellforsch Mikrosk Anat. 1968;86(1):14–25. doi: 10.1007/BF00340357. [DOI] [PubMed] [Google Scholar]
- Zambrano D., De Robertis E. Ultrastructure of the peptidergic and monoaminergic neurons in the hypothalamic neurosecretory system of anuran batracians. Z Zellforsch Mikrosk Anat. 1968;90(2):230–244. doi: 10.1007/BF00339431. [DOI] [PubMed] [Google Scholar]