Abstract
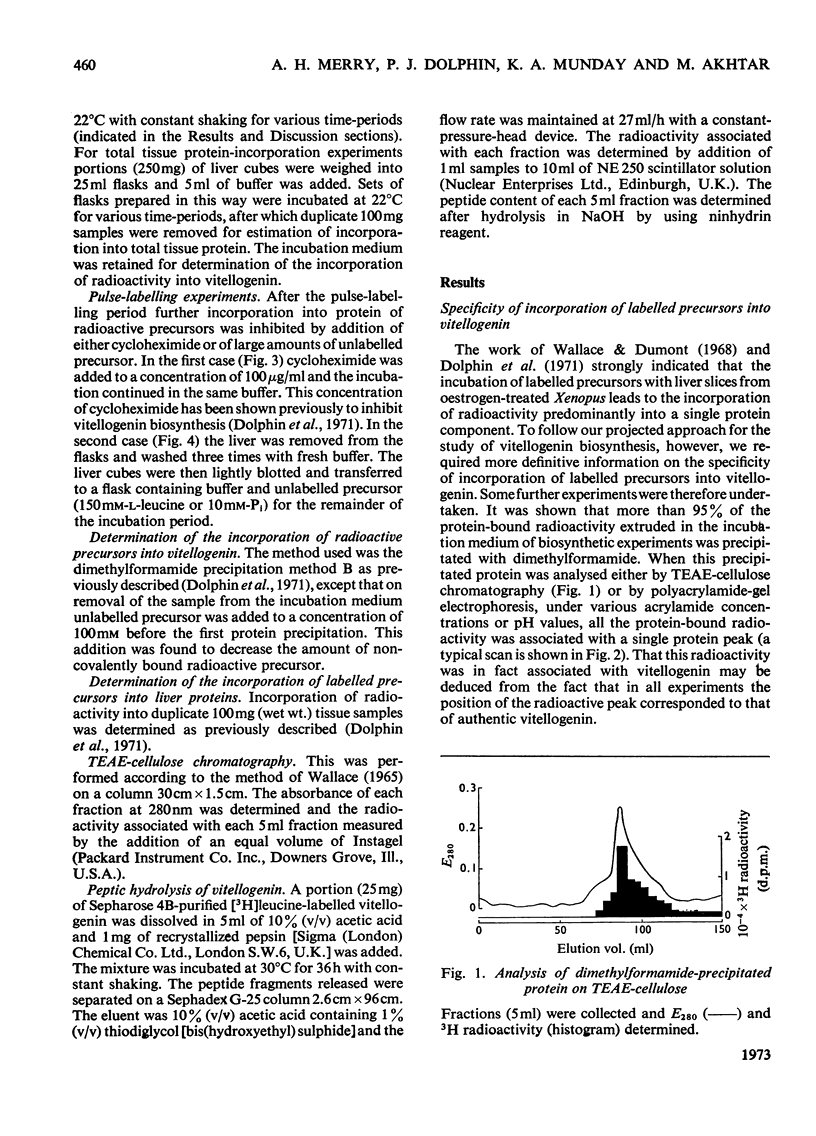
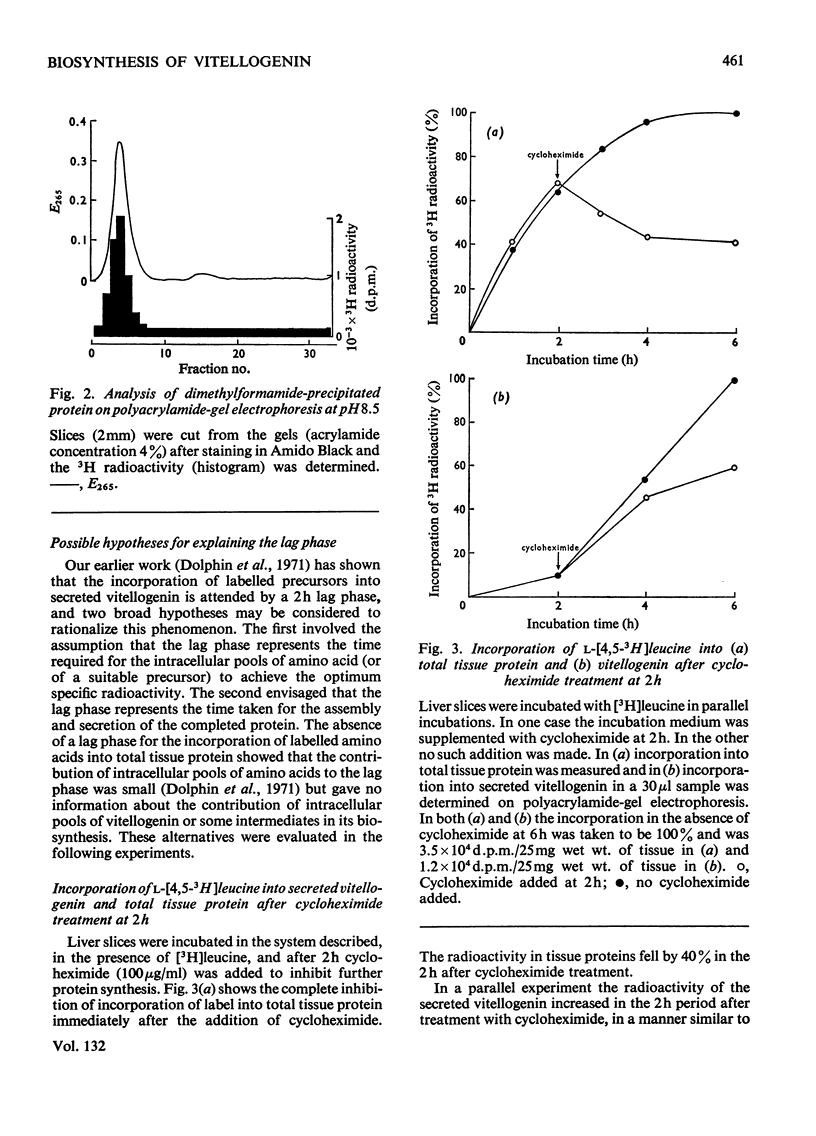
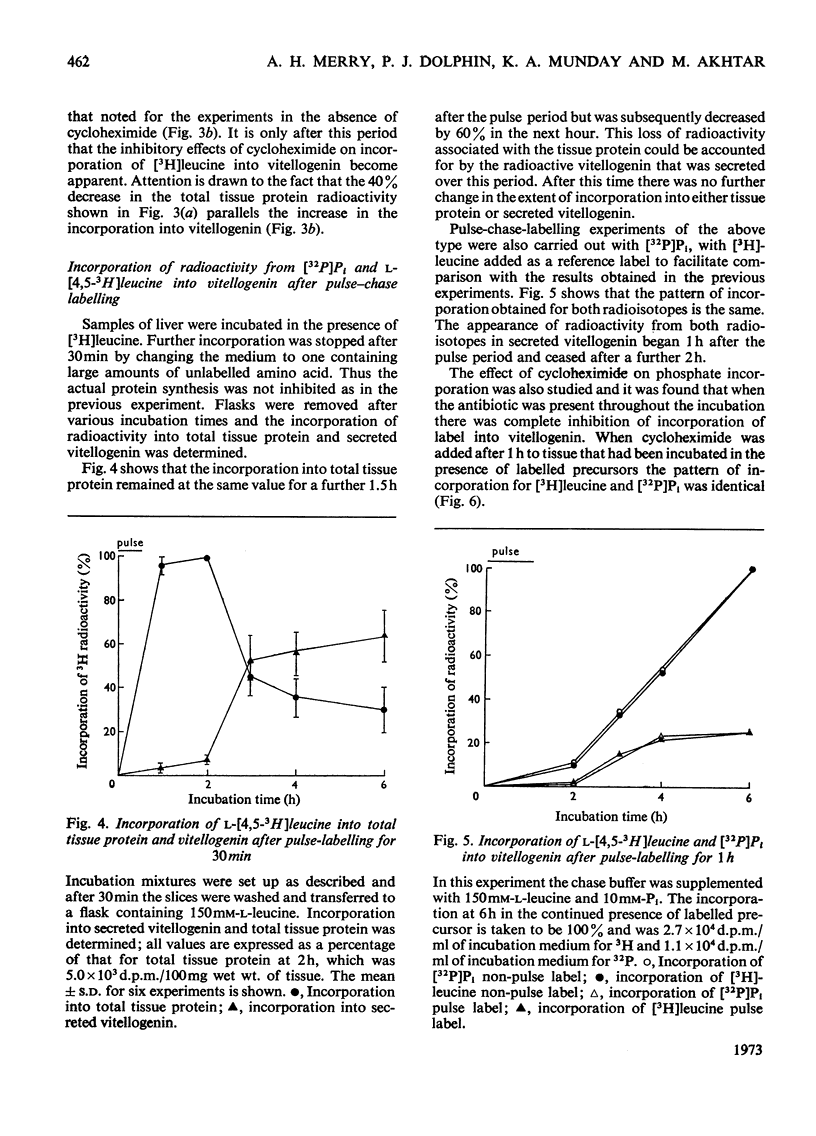
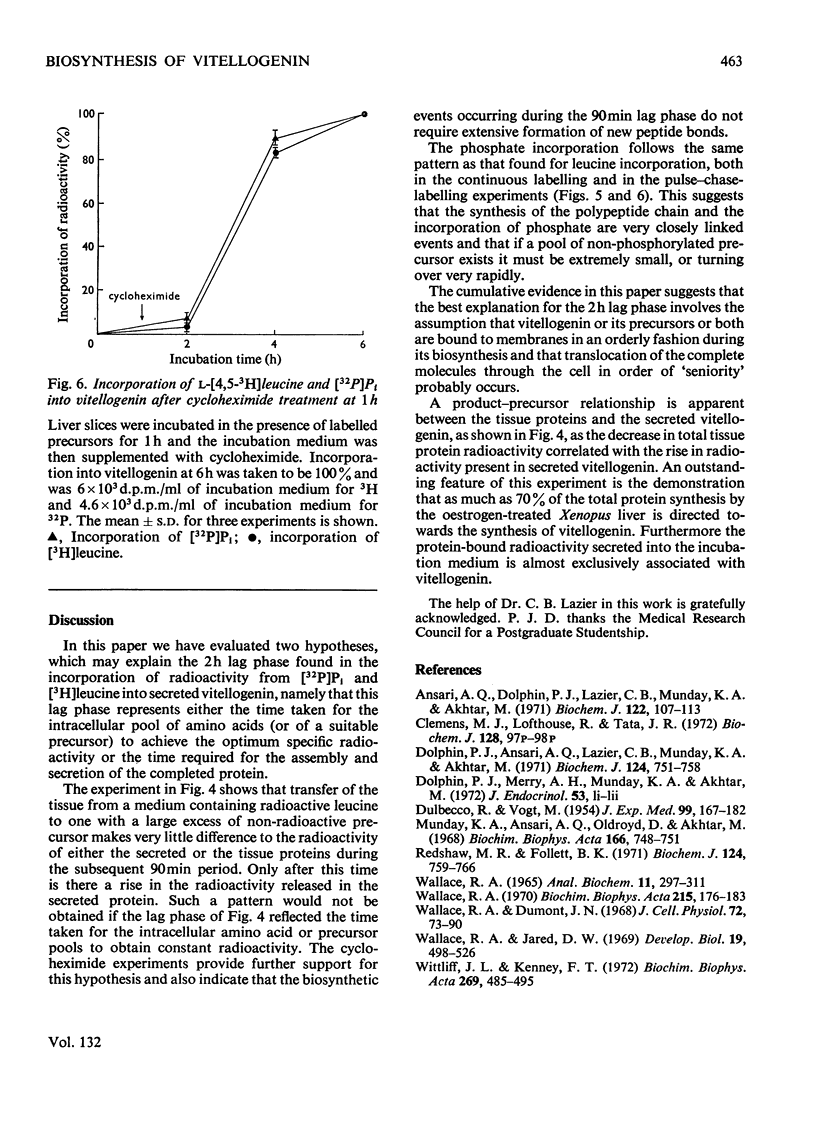
1. Incorporation of [32P]Pi and [3H]leucine into vitellogenin secreted in vitro by liver slices from oestrogen-treated Xenopus laevis is accompanied by a 2h lag; no lag is apparent for the incorporation into total tissue protein. 2. The addition of cycloheximide was found immediately to inhibit further incorporation of radioactive leucine into total tissue protein. The incorporation into secreted vitellogenin, however, continued for 2h after the addition of cycloheximide. 3. Pulse-labelling of liver slices with [3H]leucine for 30min, followed by a chase with a large excess of unlabelled leucine, resulted in the appearance of radioactivity in secreted vitellogenin from 90min after the end of the pulse period. 4. Evidence is presented which suggests that of the radioactivity from [3H]leucine incorporated into proteins by the liver of oestrogen-treated Xenopus some 70% is present in the single protein vitellogenin. 5. The incorporation of [32P]Pi into vitellogenin followed a pattern identical with that found for [3H]leucine in the pulse-labelling experiments and this indicates that synthesis of the polypeptide chain and incorporation of Pi are closely linked processes. 6. The cumulative evidence suggests that the 2h lag phase represents the time required for the assembly and secretion of this multicomponent protein.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A. Q., Dolphin P. J., Lazier C. B., Munday K. A., Akhtar M. Chemical composition of an oestrogen-induced calcium-binding glycolipophosphoprotein in Xenopus laevis. Biochem J. 1971 Mar;122(1):107–113. doi: 10.1042/bj1220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin P. J., Ansari A. Q., Lazier C. B., Munday K. A., Akhtar M. Studies on the induction and biosynthesis of vitellogenin, an oestrogen-induced glycolipophosphoprotein. Biochem J. 1971 Oct;124(4):751–758. doi: 10.1042/bj1240751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday K. A., Ansari A. Q., Oldroyd D., Akhtar M. Oestrogen-induced calcium-binding protein in Xenopus laevis. Biochim Biophys Acta. 1968 Oct 29;166(3):748–751. doi: 10.1016/0005-2787(68)90393-6. [DOI] [PubMed] [Google Scholar]
- Redshaw M. R., Follett B. K. The crystalline yolk-platelet proteins and their soluble plasma precursor in an amphibian, Xenopus laevis. Biochem J. 1971 Oct;124(4):759–766. doi: 10.1042/bj1240759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swearingen K. C., Nicoll C. S. Prolactin turnover in rat adenohypophyses in vivo: its evaluation as a method for estimating secretion rates. J Endocrinol. 1972 Apr;53(1):1–15. doi: 10.1677/joe.0.0530001. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Dumont J. N. The induced synthesis and transport of yolk proteins and their accumulation by the oocyte in Xenopus laevis. J Cell Physiol. 1968 Oct;72(2 Suppl):73–89. doi: 10.1002/jcp.1040720407. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W. Studies on amphibian yolk. 8. The estrogen-induced hepatic synthesis of a serum lipophosphoprotein and its selective uptake by the ovary and trasformation into yolk platelet proteins in Xenopus laevis. Dev Biol. 1969 May;19(5):498–526. doi: 10.1016/0012-1606(69)90085-2. [DOI] [PubMed] [Google Scholar]
- Wallace R. A. Resolution and isolation of avian and amphibian yolk-granule proteins using TEAE-cellulose. Anal Biochem. 1965 May;11(2):297–311. doi: 10.1016/0003-2697(65)90018-7. [DOI] [PubMed] [Google Scholar]
- Wallace R. A. Studies on amphibian yolk. IX. Xenopus vitellogenin. Biochim Biophys Acta. 1970 Jul 21;215(1):176–183. doi: 10.1016/0304-4165(70)90400-9. [DOI] [PubMed] [Google Scholar]
- Wittliff J. L., Kenney F. T. Regulation of yolk protein synthesis in amphibian liver. I. Induction of lipovitellin synthesis by estrogen. Biochim Biophys Acta. 1972 May 29;269(3):485–492. doi: 10.1016/0005-2787(72)90136-0. [DOI] [PubMed] [Google Scholar]


