Abstract
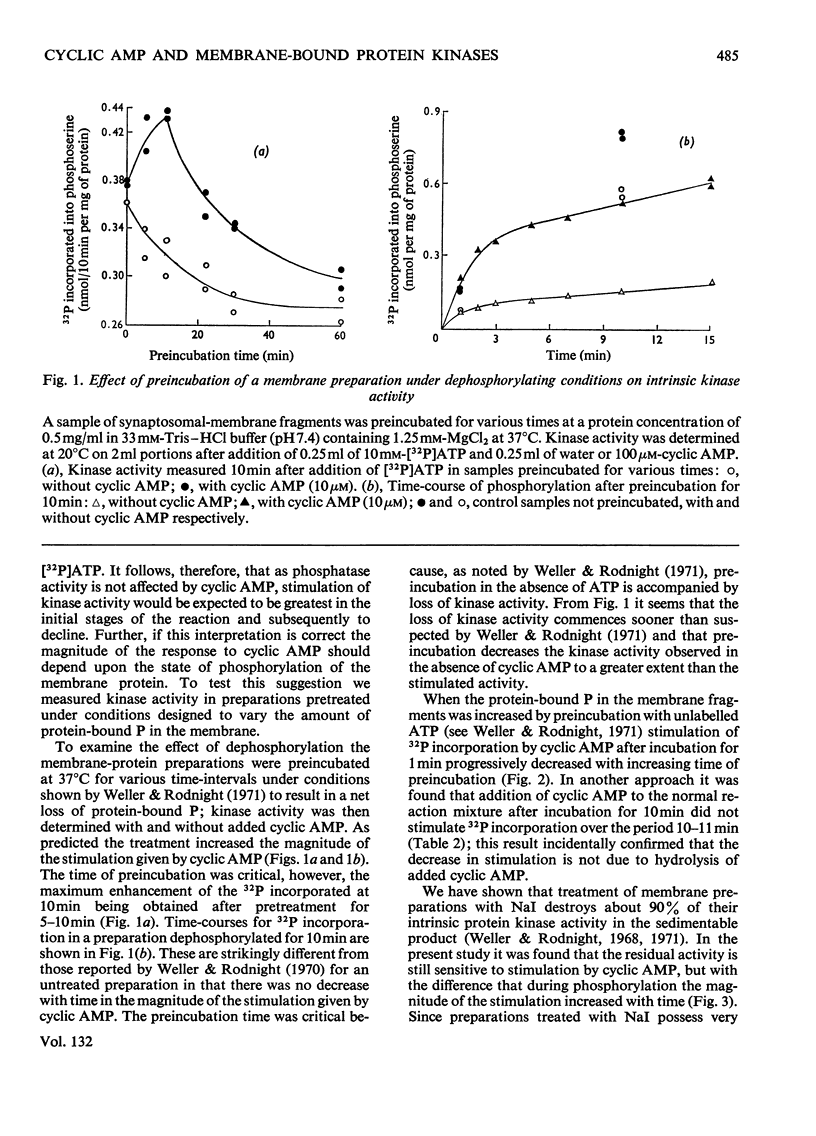
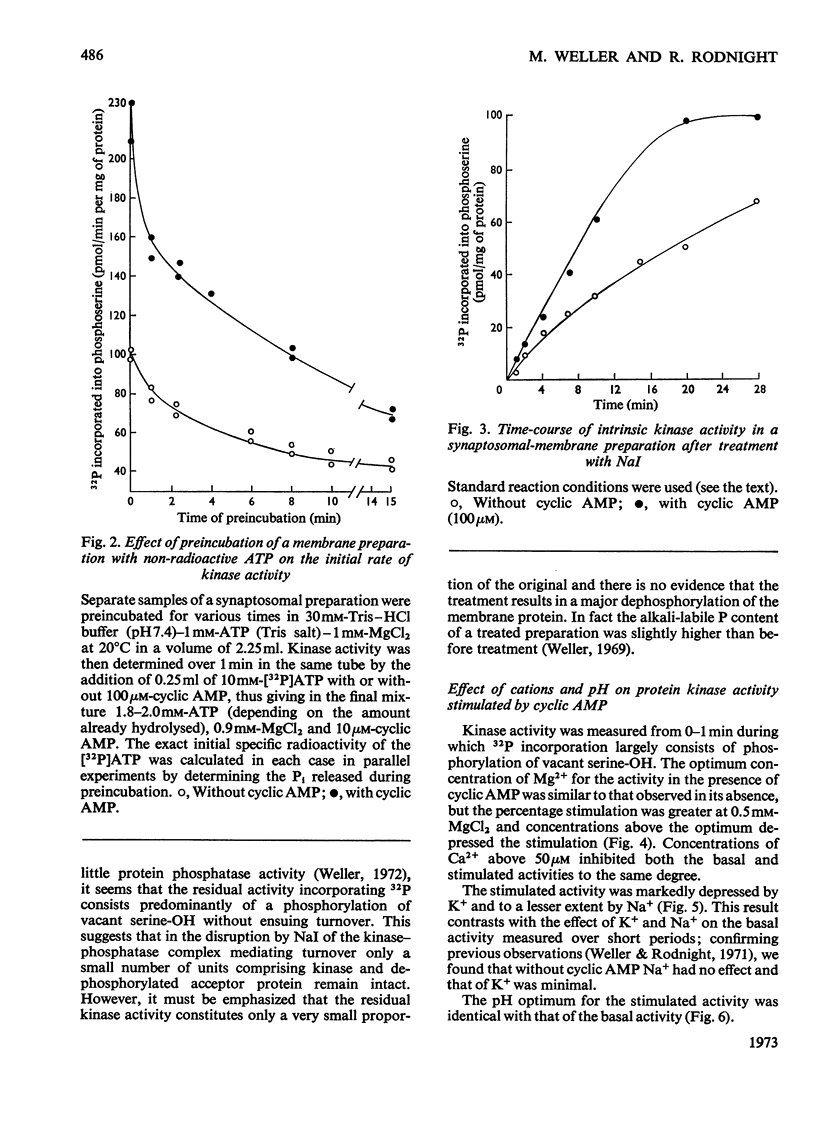
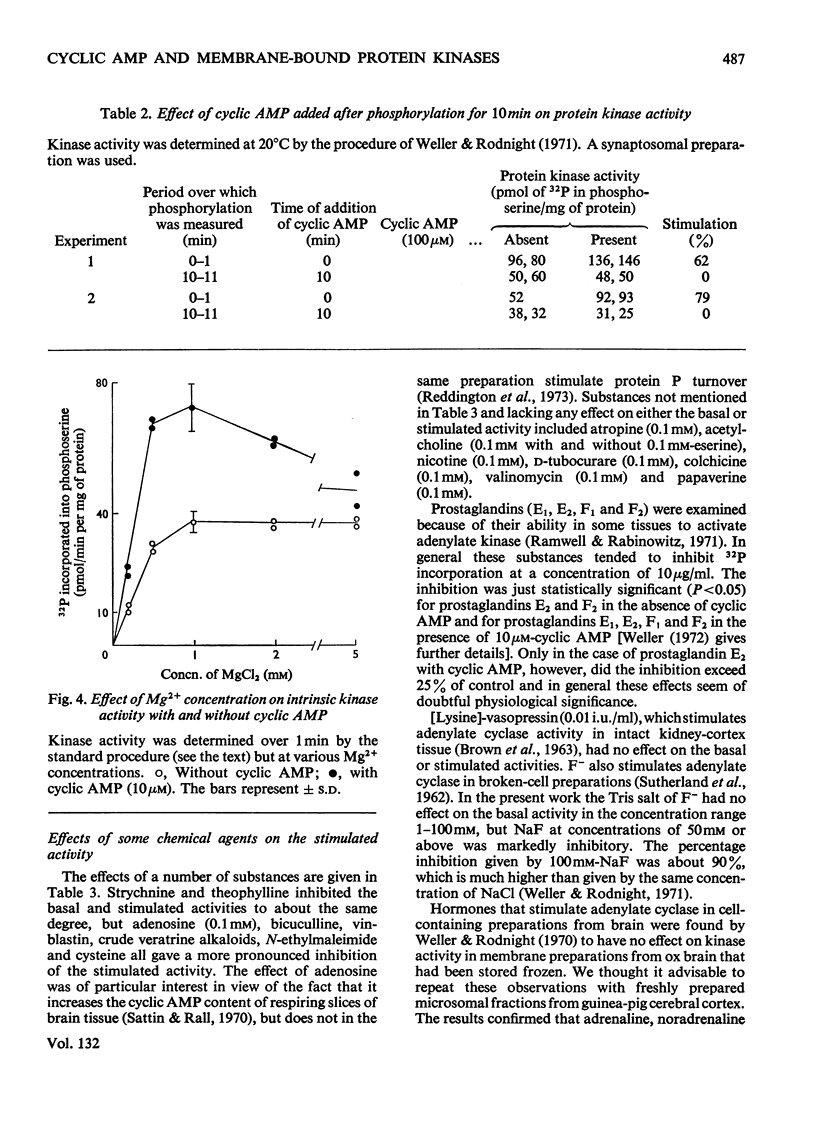
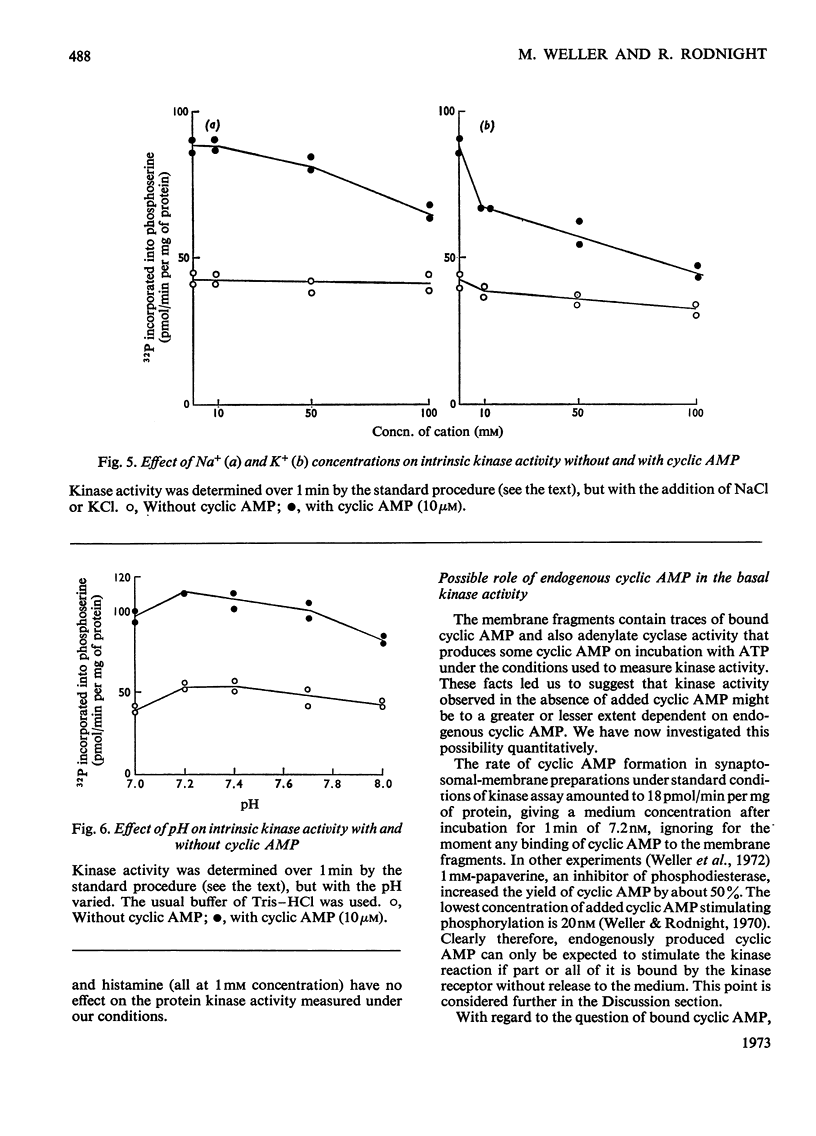
1. Properties of the stimulation by cyclic AMP of the intrinsic protein kinase activity of membrane fragments from ox brain were studied. 2. Stimulation of activity declined from about 100% at 1min to less than 20% at 10min. The time-course was explained by the observation that cyclic AMP did not stimulate turnover of protein-bound serine phosphate once the membrane protein was fully phosphorylated. 3. Cyclic AMP accelerated the activity of a component of the basal activity rather than activating a different kinase. 4. The pH optimum for both the stimulated and basal activities was 7.2–7.4. NaCl (100mm) and KCl (10–100mm) inhibited the stimulated activity but did not affect the basal activity. 5. Strychnine and theophylline inhibited both activites equally, but the stimulated activity was more sensitive to inhibition by adenosine, bicuculline, vinblastin, veratrine, N-ethylmaleimide and cysteine. 6. No firm evidence for a role for endogenous cyclic AMP in the basal activity was found, but the possibility was not excluded. 7. Some 90% of both the stimulated and basal activities remained in an insoluble form after treatment of the membrane fragments with Triton X-100 (0.5%).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN E., CLARKE D. L., ROUX V., SHERMAN G. H. The stimulation of adenosine 3,5-monophosphate production by antidiuretic factors. J Biol Chem. 1963 Feb;238:852–853. [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3'.5'-nucleotide phosphodiesterase. Effect of binding protein on the hydrolysis of cyclic AMP. Biochem Biophys Res Commun. 1972 Jan 14;46(1):99–105. doi: 10.1016/0006-291x(72)90635-3. [DOI] [PubMed] [Google Scholar]
- Decsi L., Rodnight R. The phosvitin kinase enzyme of cerebral microsomes. J Neurochem. 1965 Sep-Oct;12(9):791–796. doi: 10.1111/j.1471-4159.1965.tb10263.x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEALD P. J. The incorporation of phosphate into cerebral phosphoportein promoted by electrical impulses. Biochem J. 1957 Aug;66(4):659–663. doi: 10.1042/bj0660659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Maeno H., Greengard P. Phosphorylation of endogenous protein of rat brain by cyclic adenosine 3',5'-monophosphate-dependent protein kinase. J Biol Chem. 1971 Dec 25;246(24):7731–7739. [PubMed] [Google Scholar]
- Jones D. A., Rodnight R. Protein-bound phosphorylserine in acid hydrolysates of brain tissue. The determination of ( 32 P)phosphorylserine by ion-exchange chromatography and electrophoresis. Biochem J. 1971 Feb;121(4):597–600. doi: 10.1042/bj1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W., McIlwain H. The effect of electrical stimulation upon the accumulation of adenosine 3',5'-phosphate in isolated cerebral tissue. J Neurochem. 1969 Apr;16(4):485–491. doi: 10.1111/j.1471-4159.1969.tb06847.x. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Greengard P. Dopamine-sensitive adenyl cyclase: possible role in synaptic transmission. Science. 1971 Dec 24;174(4016):1346–1349. doi: 10.1126/science.174.4016.1346. [DOI] [PubMed] [Google Scholar]
- Libet B., Tosaka T. Dopamine as a synaptic transmitter and modulator in sympathetic ganglia: a different mode of synaptic action. Proc Natl Acad Sci U S A. 1970 Oct;67(2):667–673. doi: 10.1073/pnas.67.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno H., Johnson E. M., Greengard P. Subcellular distribution of adenosine 3',5'-monophosphate-dependent protein kinase in rat brain. J Biol Chem. 1971 Jan 10;246(1):134–142. [PubMed] [Google Scholar]
- RABINOWITZ M., LIPMANN F. Reversible phosphate transfer between yolk phosphoprotein and adenosine triphosphate. J Biol Chem. 1960 Apr;235:1043–1050. [PubMed] [Google Scholar]
- ROSE S. P., HEALD P. J. A phosphoprotein phosphatase from ox brain. Biochem J. 1961 Nov;81:339–347. doi: 10.1042/bj0810339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Reddington M., Rodnight R., Williams M. Turnover of protein-bound serine phosphate in respiring slices of guinea-pig cerebral cortex. Effects of putative transmitters, tetrodotoxin and other agents. Biochem J. 1973 Mar;132(3):475–482. doi: 10.1042/bj1320475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison G. A., Schmidt M. J., Sutherland E. W. On the development and properties of the brain adenyl cyclase system. Adv Biochem Psychopharmacol. 1970;3:11–30. [PubMed] [Google Scholar]
- Rodnight R., Lavin B. E. Enzyme transfer of phosphate from adenosine triphosphate to protein-bound serine residues in cerebral microsomes. Biochem J. 1966 Nov;101(2):495–501. doi: 10.1042/bj1010495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnight R., Lavin B. E. Phosvitin kinase from brain: activation by ions and subcellular distribution. Biochem J. 1964 Oct;93(1):84–91. doi: 10.1042/bj0930084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnight R. The effect of chemical agents on the turnover of the bound phosphate associated with the sodium-and-potassium ion-stimulated adenosine triphosphatase in ox brain microsomes. Biochem J. 1970 Nov;120(1):1–13. doi: 10.1042/bj1200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnight R., Weller M., Goldfarb P. S. Large scale preparation of a crude membrane fraction from ox brain. J Neurochem. 1969 Dec;16(12):1591–1597. doi: 10.1111/j.1471-4159.1969.tb10357.x. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Sattin A., Rall T. W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3', 5'-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970 Jan;6(1):13–23. [PubMed] [Google Scholar]
- Siggins G. R., Hoffer B. J., Bloom F. E. Studies on norepinephrine-containing afferents to Purkinje cells of rat cerebellum. 3. Evidence for mediation of norepinephrine effects by cyclic 3',5'-adenosine monophosphate. Brain Res. 1971 Feb 5;25(3):535–553. doi: 10.1016/0006-8993(71)90459-8. [DOI] [PubMed] [Google Scholar]
- TREVOR A. J., RODNIGHT R. THE SUBCELLULAR LOCALIZATION OF CEREBRAL PHOSPHOPROTEINS SENSITIVE TO ELECTRICAL STIMULATION. Biochem J. 1965 Jun;95:889–896. doi: 10.1042/bj0950889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller M., Rodnight R., Carrera D. Determination of adenosine 3':5'-cyclic monophosphate in cerebral tissues by saturation analysis. Assessment of a method using a binding protein from ox muscle. Biochem J. 1972 Aug;129(1):113–121. doi: 10.1042/bj1290113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller M., Rodnight R. Stimulation by cyclic AMP of intrinsic protein kinase activity in ox brain membrane preparations. Nature. 1970 Jan 10;225(5228):187–188. doi: 10.1038/225187a0. [DOI] [PubMed] [Google Scholar]
- Weller M., Rodnight R. Turnover of protein-bound phosphorylserine in membrane preparations from ox brain catalysed by intrinsic kinase and phosphatase activity. Biochem J. 1971 Sep;124(2):393–406. doi: 10.1042/bj1240393. [DOI] [PMC free article] [PubMed] [Google Scholar]


