Abstract
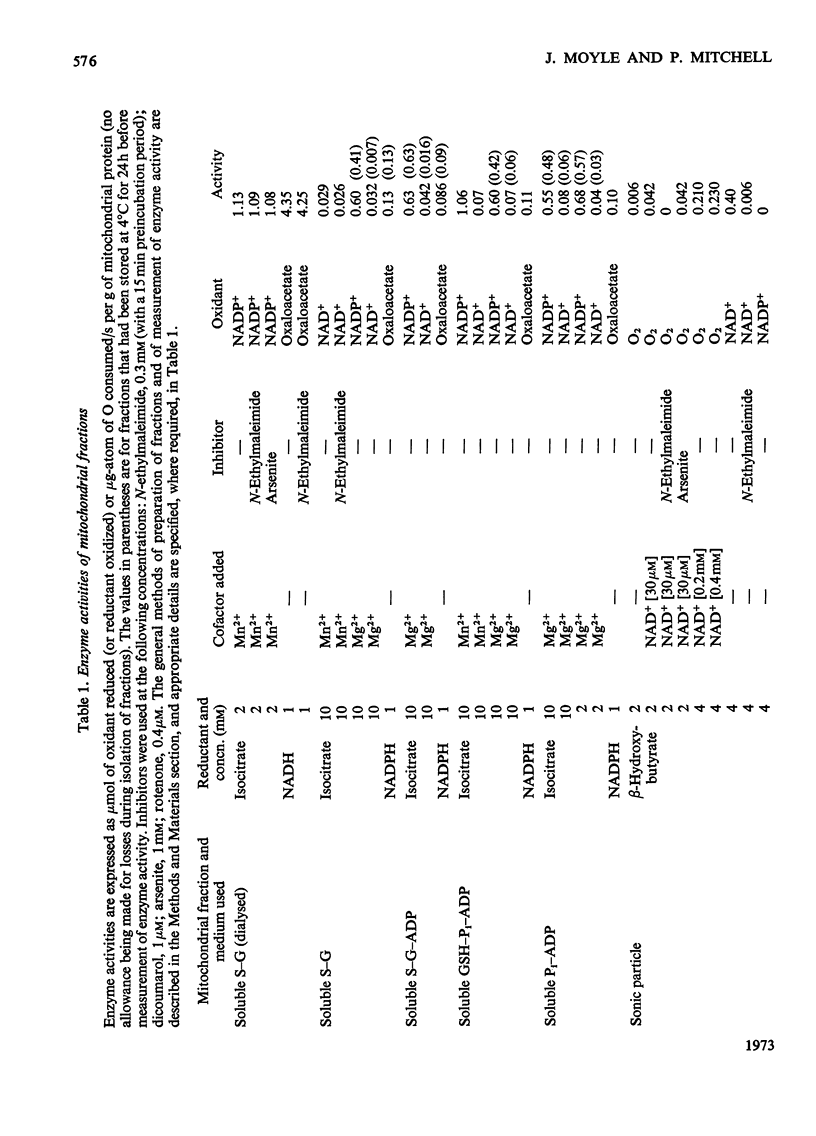
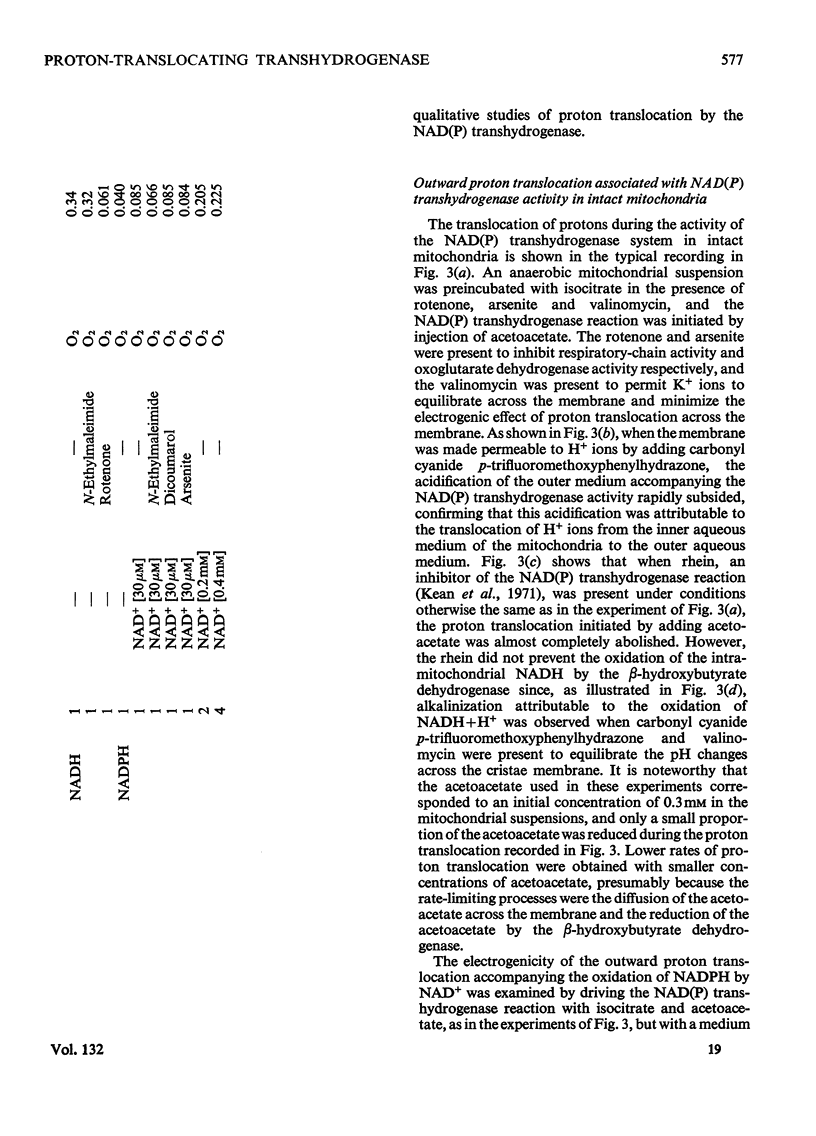
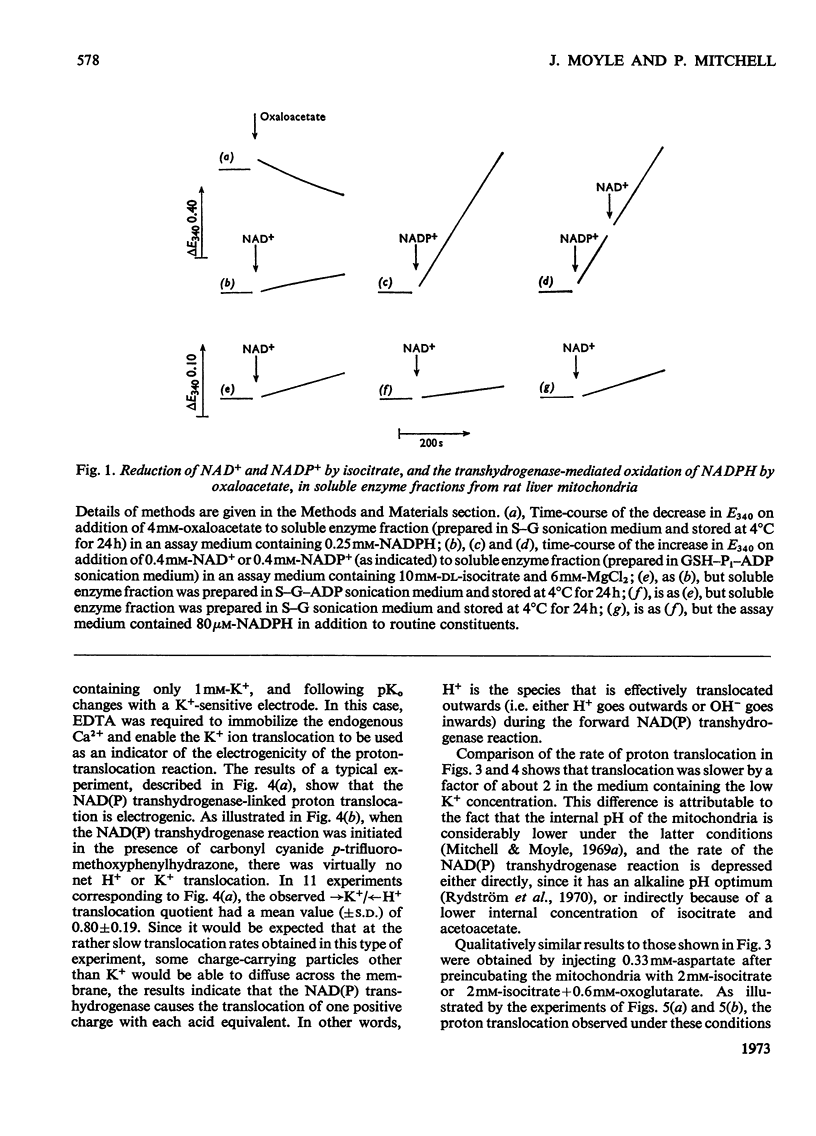
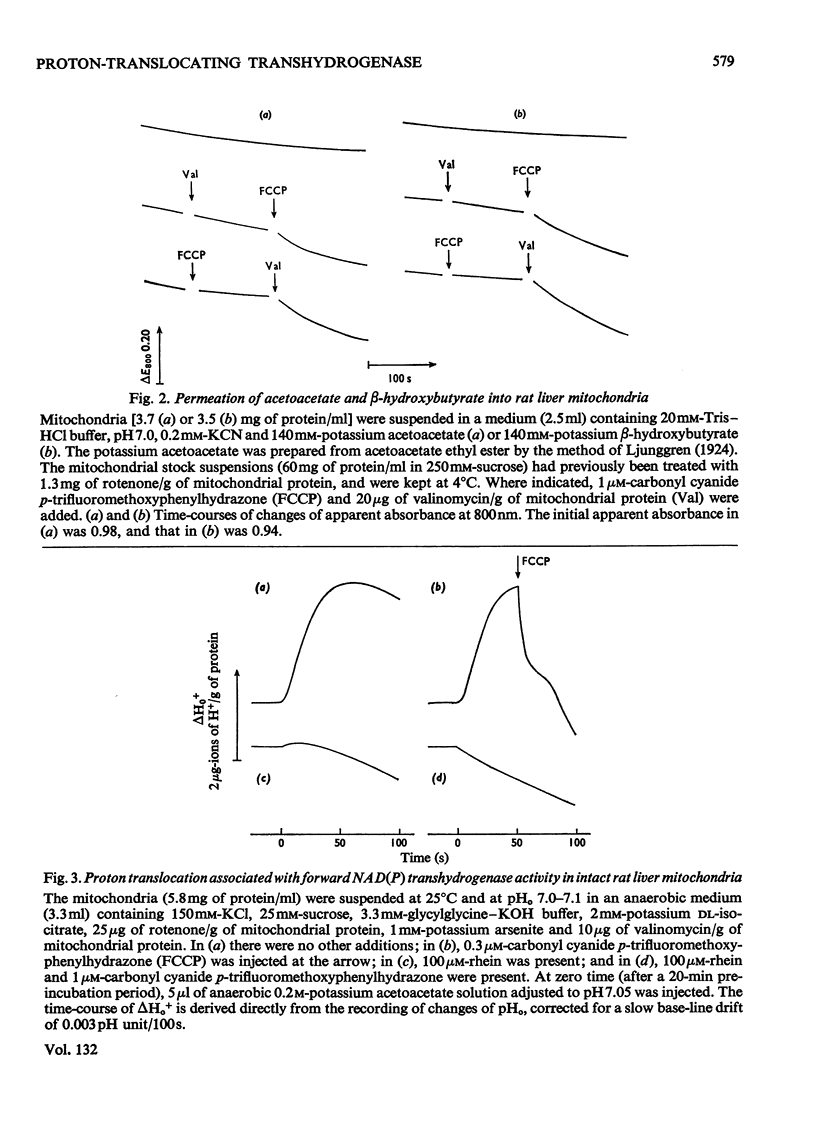
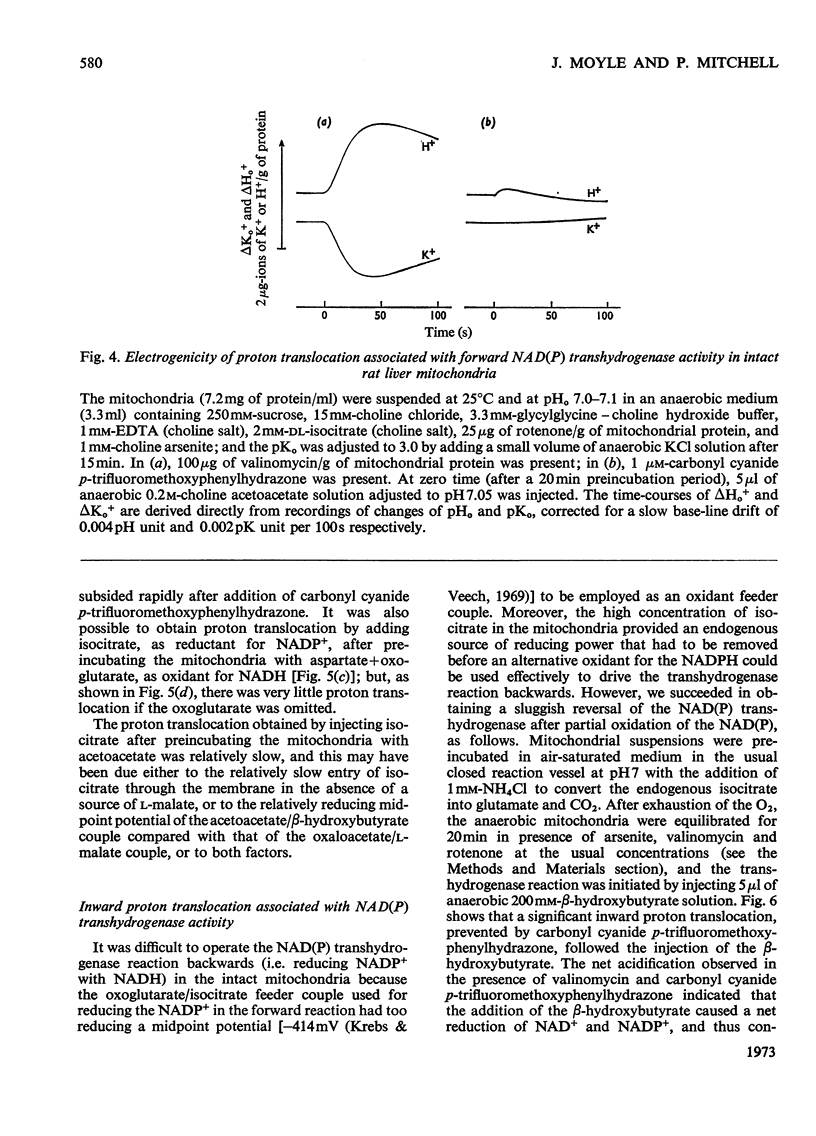
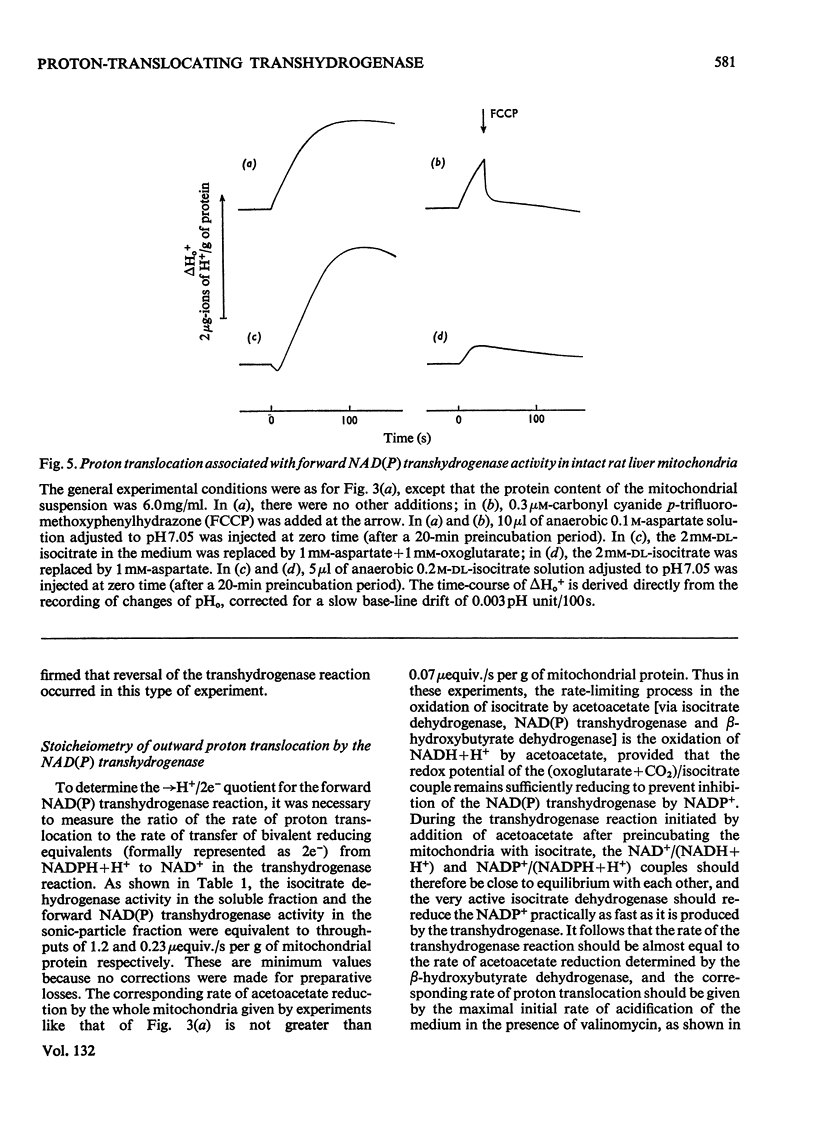
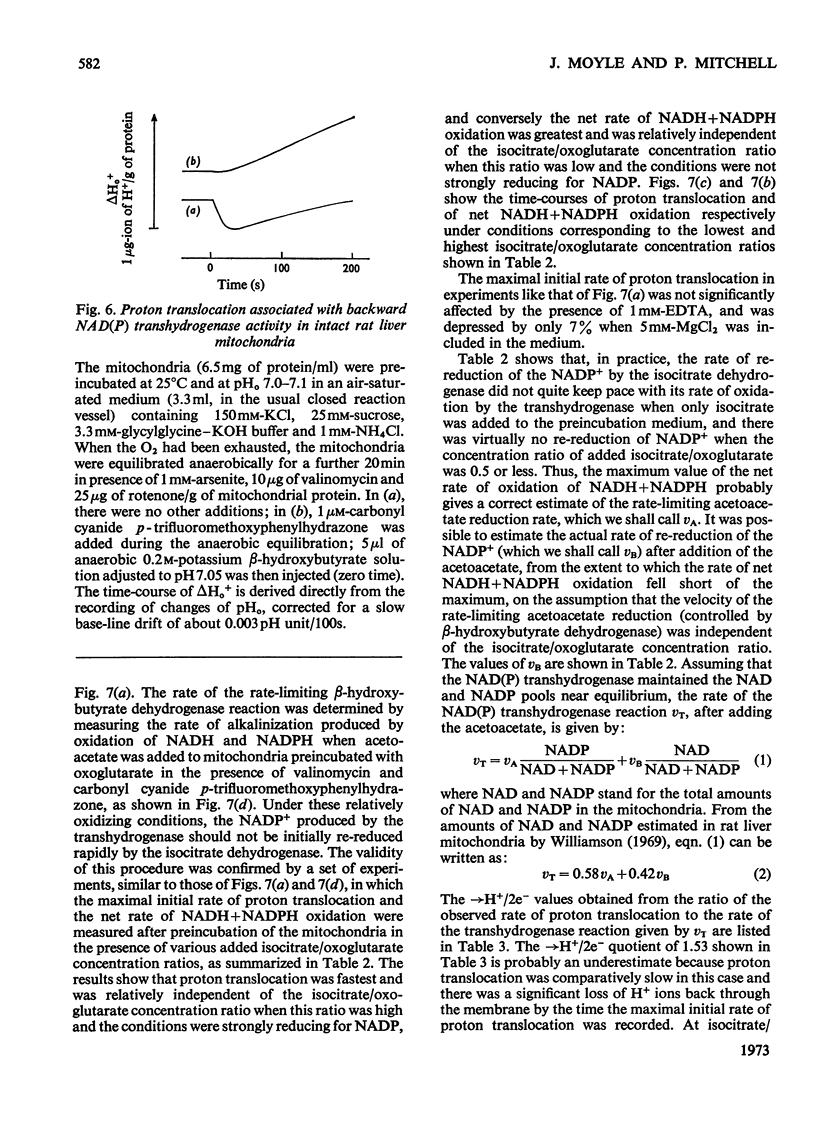
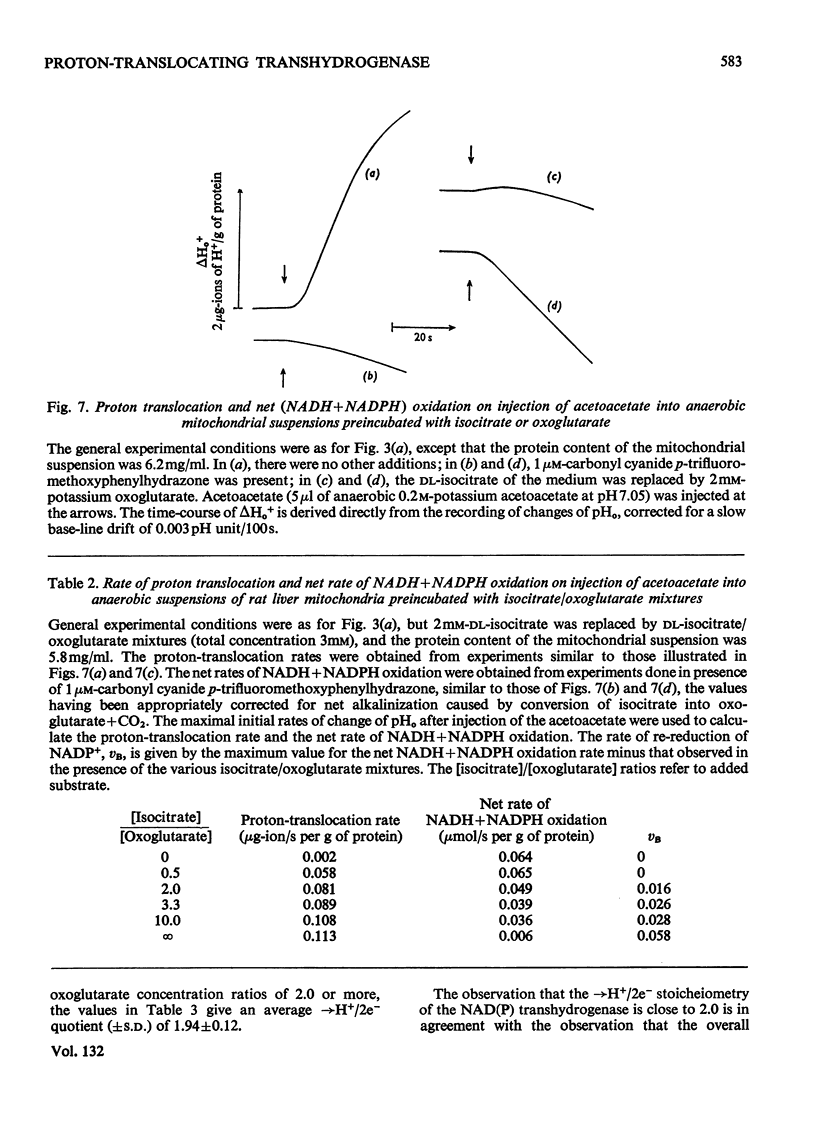
1. The NAD(P) transhydrogenase activity of the soluble fraction of sonicated rat liver mitochondrial preparations was greater than the NAD-linked isocitrate dehydrogenase activity, and the NAD-linked and NADP-linked isocitrate dehydrogenase activities were not additive. The NAD-linked isocitrate dehydrogenase activity was destroyed by an endogenous autolytic system or by added nucleotide pyrophosphatase, and was restored by a catalytic amount of NADP. 2. We concluded that the isocitrate dehydrogenase of rat liver mitochondria was exclusively NADP-specific, and that the oxoglutarate/isocitrate couple could therefore be used unequivocally as redox reactant for NADP in experiments designed to operate only the NAD(P) transhydrogenase (or loop 0) segment of the respiratory chain in intact mitochondria. 3. During oxidation of isocitrate by acetoacetate in intact, anaerobic, mitochondria via the rhein-sensitive, but rotenone- and arsenite-insensitive, NAD(P) transhydrogenase, measurements of the rates of carbonyl cyanide p-trifluoromethoxyphenylhydrazone-sensitive and carbonyl cyanide p-trifluoromethoxyphenylhydrazone-insensitive pH change in the presence of various oxoglutarate/isocitrate concentration ratios gave an →H+/2e− quotient of 1.94±0.12 for outward proton translocation by the NAD(P) transhydrogenase. 4. Measurements with a K+-sensitive electrode confirmed that the electrogenicity of the NAD(P) transhydrogenase reaction corresponded to the translocation of one positive charge per acid equivalent. 5. Sluggish reversal of the NAD(P) transhydrogenase reaction resulted in a significant inward proton translocation. 6. The possibility that isocitrate might normally be oxidized via loop 0 at a redox potential of −450mV, or even more negative, is discussed, and implies that a P/O quotient of 4 for isocitrate oxidation might be expected.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A., Azzone G. F. Swelling and shrinkage phenomena in liver mitochondria. VI. Metabolism-independent swelling coupled to ion movement. Biochim Biophys Acta. 1967 May 9;131(3):468–478. doi: 10.1016/0005-2728(67)90006-0. [DOI] [PubMed] [Google Scholar]
- Cohen P. T., Kaplan N. O. Kinetic characteristics of the pyridine nucleotide transhydrogenase from Pseudomonas aeruginosa. J Biol Chem. 1970 Sep 25;245(18):4666–4672. [PubMed] [Google Scholar]
- Da Cruz A. T., Rydström J., Ernster L. Steady-state kinetics of mitochondrial nicotinamide nucleotide transhydrogenase. 1. The nonenergy-linked reaction. Eur J Biochem. 1971 Nov 11;23(2):203–211. doi: 10.1111/j.1432-1033.1971.tb01610.x. [DOI] [PubMed] [Google Scholar]
- Dontsov A. E., Grinius L. L., Jasaitis A. A., Severina I. I., Skulachev V. P. A study on the mechanism of energy coupling in the redox chain. I. Transhydrogenase: the fourth site of the redox chain energy coupling. J Bioenerg. 1972 Jun;3(3):277–303. doi: 10.1007/BF01515975. [DOI] [PubMed] [Google Scholar]
- ERNSTER L., GLASKY A. J. On the mitochondrial oxidation of isocitrate. Biochim Biophys Acta. 1960 Feb 12;38:168–170. doi: 10.1016/0006-3002(60)91216-6. [DOI] [PubMed] [Google Scholar]
- ERNSTER L., NAVAZIO F. Studies on TPN-linked oxidations. I. Pathways of isocitrate oxidation in rat liver micochondria. Biochim Biophys Acta. 1957 Nov;26(2):408–415. doi: 10.1016/0006-3002(57)90023-9. [DOI] [PubMed] [Google Scholar]
- ERNSTER L., NAVAZIO F. The cytoplasmic distribution of isocitric dehydrogenases. Exp Cell Res. 1956 Aug;11(2):483–486. doi: 10.1016/0014-4827(56)90124-0. [DOI] [PubMed] [Google Scholar]
- GOEBELL H., KLINGENBERG M. DPN-SPEZIFISCHE ISOCITRAT-DEHYDROGENASE DER MITOCHONDRIEN. I. KINETISCHE EIGENSSCHAFTEN, VORKOMMEN UND FUNKTION DER DPN-SPEZIFISCHEN ISOCITRAT-DEHYDROGENASE. Biochem Z. 1964 Sep 28;340:441–464. [PubMed] [Google Scholar]
- Garland P. B. Control of citrate synthesis in mitochondria. Biochem Soc Symp. 1968;27:41–60. [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Kean E. A., Gutman M., Singer T. P. Studies on the respiratory chain-linked nicotinamide adenine dinucleotide dehydrogenase. XXII. Rhein, a competitive inhibitor of the dehydrogenase. J Biol Chem. 1971 Apr 25;246(8):2346–2353. [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- LEHNINGER A. L., SUDDUTH H. C., WISE J. B. D-beta-Hydroxybutyric dehydrogenase of muitochondria. J Biol Chem. 1960 Aug;235:2450–2455. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in energy transduction: a logical development of biochemical knowledge. J Bioenerg. 1972 May;3(1):5–24. doi: 10.1007/BF01515993. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Evidence discriminating between the chemical and the chemiosmotic mechanisms of electron transport phosphorylation. Nature. 1965 Dec 18;208(5016):1205–1206. doi: 10.1038/2081205a0. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Proton translocation coupled to ATP hydrolysis in rat liver mitochondria. Eur J Biochem. 1968 May;4(4):530–539. doi: 10.1111/j.1432-1033.1968.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Respiration-driven proton translocation in rat liver mitochondria. Biochem J. 1967 Dec;105(3):1147–1162. doi: 10.1042/bj1051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Translocation of some anions cations and acids in rat liver mitochondria. Eur J Biochem. 1969 Jun;9(2):149–155. doi: 10.1111/j.1432-1033.1969.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Papa S., Lofrumento N. E., Kanduc D., Paradies G., Quagliariello E. The transport of citric-acid-cycle intermediates in rat-liver mitochondria. Electrical nature and coupling of the exchange-diffusion reactions with proton translocation. Eur J Biochem. 1971 Sep 13;22(1):134–143. doi: 10.1111/j.1432-1033.1971.tb01524.x. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Williams G. R., Halperin M. L., Leznoff C. C. Factors affecting the kinetics and equilibrium of exchange reactions of the citrate-transporting system of rat liver mitochondria. J Biol Chem. 1971 Sep 10;246(17):5280–5286. [PubMed] [Google Scholar]
- Rydström J., Da Cruz A. T., Ernster L. Steady-state kinetics of mitochondrial nicotinamide nucleotide transhydrogenase. 2. The energy-linked reaction. Eur J Biochem. 1971 Nov 11;23(2):212–219. doi: 10.1111/j.1432-1033.1971.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Rydström J., da Cruz A. T., Ernster L. Factors governing the kinetics and steady state of the mitochondrial nicotinamide nucleotide transhydrogenase system. Eur J Biochem. 1970 Nov;17(1):56–62. doi: 10.1111/j.1432-1033.1970.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Respiration-driven proton translocation in Micrococcus denitrificans. J Bioenerg. 1971 Sep;1(3):309–323. doi: 10.1007/BF01516290. [DOI] [PubMed] [Google Scholar]
- Skulachev V. P. Electric fields in coupling membranes. FEBS Lett. 1970 Dec 18;11(5):301–308. doi: 10.1016/0014-5793(70)80554-3. [DOI] [PubMed] [Google Scholar]
- Stein A. M., Stein J. H., Kirkman S. K. Diphosphopyridine nucleotide specific isocitric dehydrogenase of mammalian mitochondria. I. On the roles of pyridine nucleotide transhydrogenase and the isocitric dehydrogenases in the respiration of mitochondria of normal and neoplastic tissues. Biochemistry. 1967 May;6(5):1370–1379. doi: 10.1021/bi00857a020. [DOI] [PubMed] [Google Scholar]
- van de Stadt R. J., Nieuwenhuis F. J., van Dam K. On the reversibility of the energy-linked transhydrogenase. Biochim Biophys Acta. 1971 Apr 6;234(1):173–176. doi: 10.1016/0005-2728(71)90143-5. [DOI] [PubMed] [Google Scholar]


