Abstract
1. Glutathione reductase and glutathione–cystine transhydrogenase activity in supernatant fractions of whole homogenates and homogenates of mucosal and muscular layers were determined in developing rat intestine after determination of the optimum conditions for assay of the two enzymes. In jejunum from adult rat, the Km values for GSSG reductase and GSH–cystine transhydrogenase activities were 0.25mm-GSSG and 0.23mm-cystine respectively. 2. The two activities could be differentiated by stability studies since GSSG reductase was stable at 60°C for 10min and could be stored at 4°C for 24h without loss of activity. GSH–cystine transhydrogenase, on the other hand, was denatured at 60°C and completely inactive after 24h storage at 4°C. 3. Based on calculations of total activities, both enzymes increased from the eighteenth day until the animals were young adults. 4. Total GSSG reductase activity increased at a greater rate with age than total GSH–cystine transhydrogenase activity as evidenced by activity ratios for GSH–cystine transhydrogenase/GSSG reductase of 0.44 and 0.12 in ileum from suckling and adult rats respectively, and 0.31 and 0.24 in jejunum from suckling and adult rats respectively. 5. In mucosa from adult rats GSSG reductase was more active in the ileum than in the jejunum, whereas GSH–cystine transhydrogenase activity was higher in the jejunum. 6. GSH–cystine transhydrogenase was active only in the muscle cells of the ileum of 7-day-old rats but became localized primarily in the mucosal layer in the adult rat. However, GSSG reductase activity was distributed evenly between the two layers throughout the intestine.
Full text
PDF

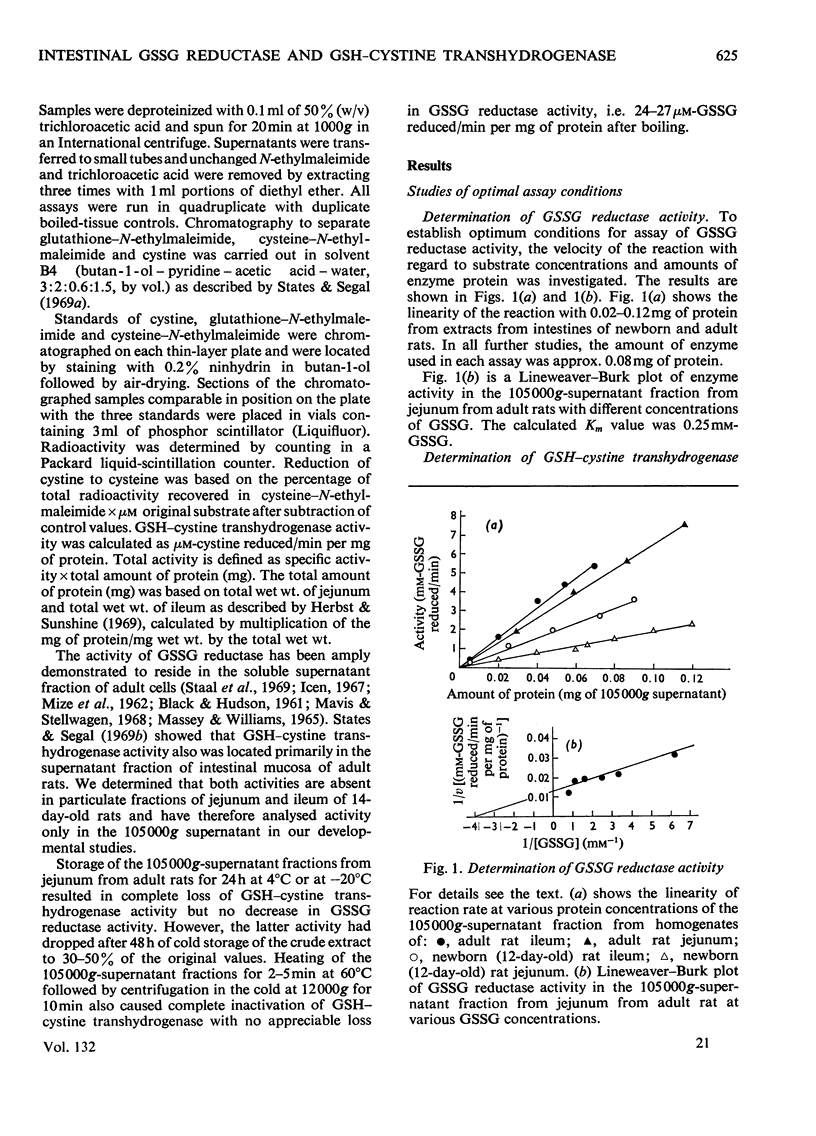
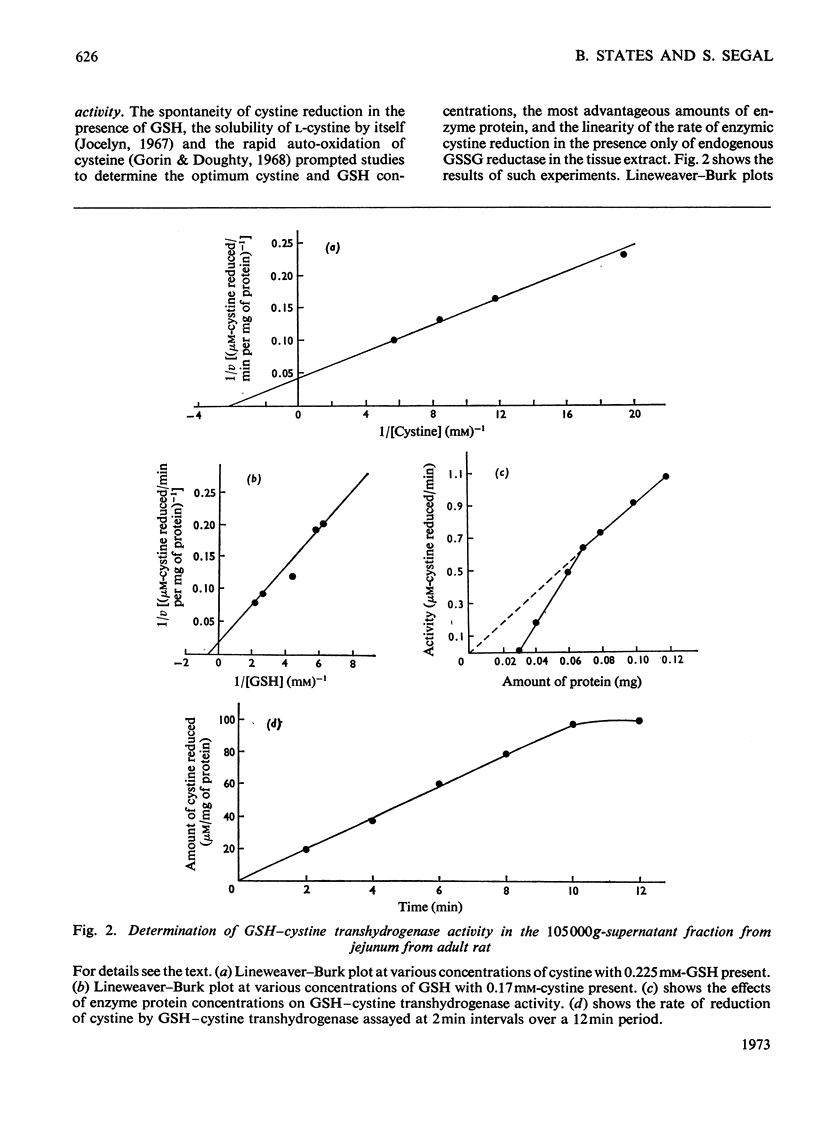

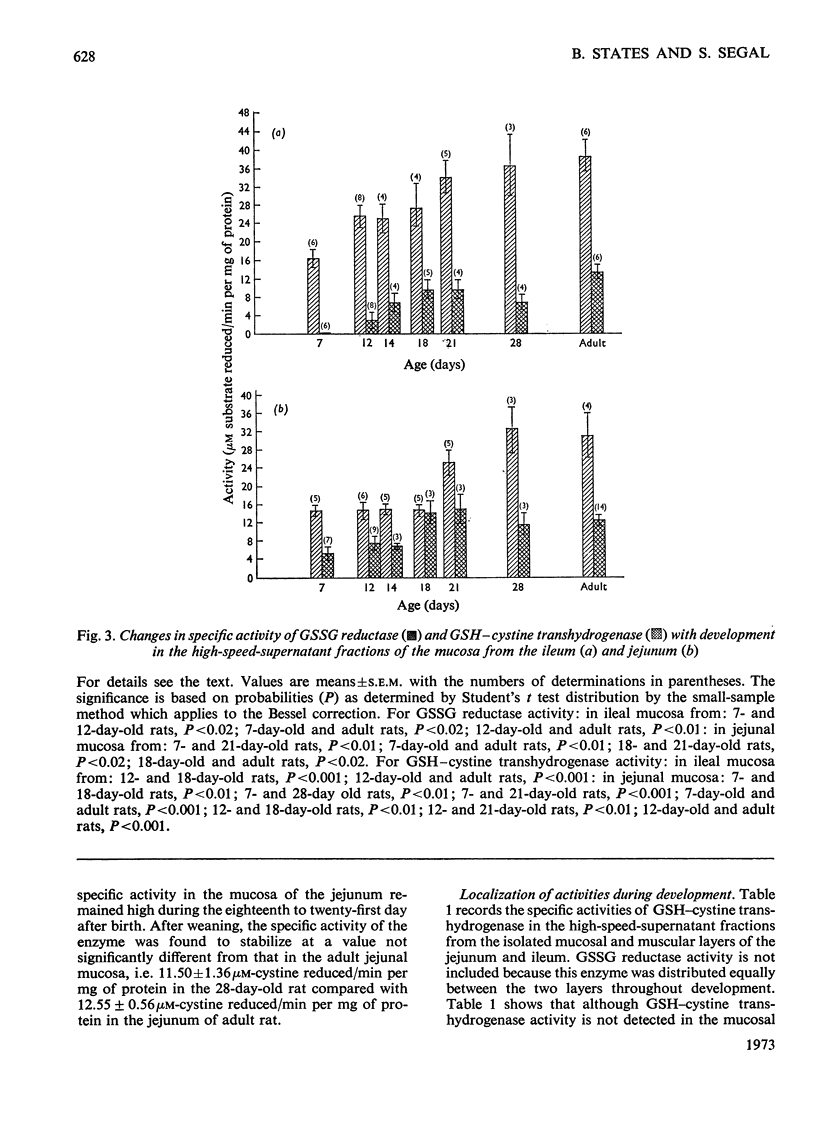
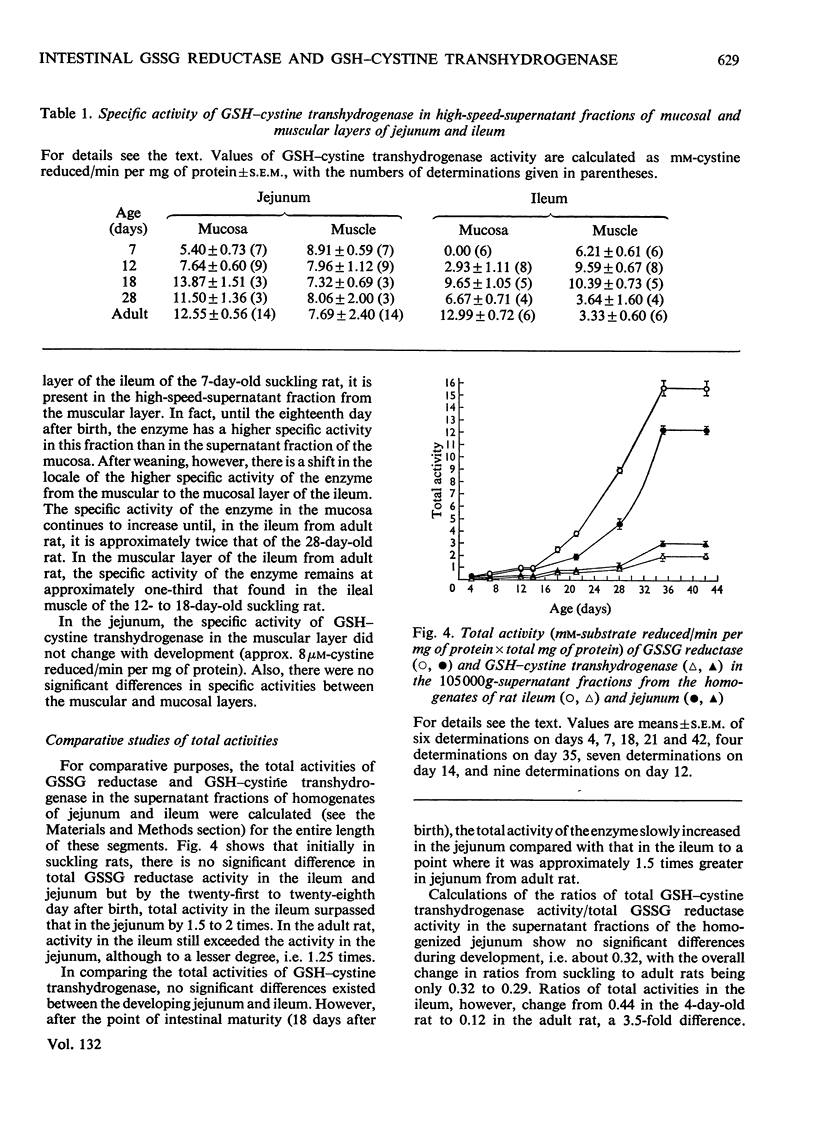


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang S. H., Wilken D. R. Participation of the unsymmetrical disulfide of coenzyme A and glutathione in an enzymatic sulfhydryl-disulfide interchange. I. Partial purification and properties of the bovine kidney enzyme. J Biol Chem. 1966 Sep 25;241(18):4251–4260. [PubMed] [Google Scholar]
- Eriksson S. A., Mannervik B. The reduction of the L-cysteine-glutathione mixed disulfide in rat liver. involvement of an enzyme catalyzing thiol-disulfide interchange. FEBS Lett. 1970 Mar 16;7(1):26–28. doi: 10.1016/0014-5793(70)80608-1. [DOI] [PubMed] [Google Scholar]
- Gorin G., Doughty G. Equilibrium constants for the reaction of glutathione with cystine and their relative oxidation-reduction potentials. Arch Biochem Biophys. 1968 Aug;126(2):547–551. doi: 10.1016/0003-9861(68)90440-2. [DOI] [PubMed] [Google Scholar]
- Herbst J. J., Sunshine P. Postnatal development of the small intestine of the rat. Changes in mucosal morphology at weaning. Pediatr Res. 1969 Jan;3(1):27–33. doi: 10.1203/00006450-196901000-00004. [DOI] [PubMed] [Google Scholar]
- Hosoda S., Nakamura W. Role of glutathione in regulation of hexose monophosphate pathway in Ehrlich ascites tumor cells. Biochim Biophys Acta. 1970 Oct 27;222(1):53–64. doi: 10.1016/0304-4165(70)90350-8. [DOI] [PubMed] [Google Scholar]
- Icén A. Glutathione reductase of human erythrocytes. Purification and properties. Scand J Clin Lab Invest Suppl. 1967;96:1–67. [PubMed] [Google Scholar]
- Jocelyn P. C. The standard redox potential of cysteine-cystine from the thiol-disulphide exchange reaction with glutathione and lipoic acid. Eur J Biochem. 1967 Oct;2(3):327–331. doi: 10.1111/j.1432-1033.1967.tb00142.x. [DOI] [PubMed] [Google Scholar]
- KOLDVSKY O., DANYSZ J., FALTOVA E., HAHN P. The postnatal proximo-distal development of glucose absorption, intestinal alkaline phosphatase activity and propulsive motility of the intestine in rats. Physiol Bohemoslov. 1963;12:208–212. [PubMed] [Google Scholar]
- Koldovský O., Heringová A., Hosková J., Jirsová V., Noack R., Friedrich M., Schenck G. The postnatal development of enzyme activities of the small intestine. Biol Neonat. 1965;9(1):33–43. doi: 10.1159/000239975. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MIZE C. E., THOMPSON T. E., LANGDON R. G. Hepatic glutathione reductase. II. Physical properties and mechanism of action. J Biol Chem. 1962 May;237:1596–1600. [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr On the reaction mechanism of yeast glutathione reductase. J Biol Chem. 1965 Nov;240(11):4470–4480. [PubMed] [Google Scholar]
- Mavis R. D., Stellwagen E. Purification and subunit structure of glutathione reductase from bakers' yeast. J Biol Chem. 1968 Feb 25;243(4):809–814. [PubMed] [Google Scholar]
- Morecki R., Paunier L., Hamilton J. R. Intestinal mucosa in cystinosis. A fine structure study. Arch Pathol. 1968 Sep;86(3):297–307. [PubMed] [Google Scholar]
- Nagai S., Black S. A thiol-disulfide transhydrogenase from yeast. J Biol Chem. 1968 Apr 25;243(8):1942–1947. [PubMed] [Google Scholar]
- Ondarza R. N., Abney R., López-Colomé A. M. Characterization of a NADPH-dependent coenzyme A-SS-glutathione reductase from yeast. Biochim Biophys Acta. 1969 Nov 4;191(2):239–248. doi: 10.1016/0005-2744(69)90243-5. [DOI] [PubMed] [Google Scholar]
- Pelichová H., Koldovský O., Heringová A., Jirsová V., Kraml J. Postnatal changes of activity and electrophoretic pattern of jejunal and ileal nonspecific esterase and alkaline phosphatase of the rat. Effect of adrenalectomy. Can J Biochem. 1967 Sep;45(9):1375–1384. doi: 10.1139/o67-162. [DOI] [PubMed] [Google Scholar]
- Pinto R. E., Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969 Mar;112(1):109–115. doi: 10.1042/bj1120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Glutathione-homocystine transhydrogenase. J Biol Chem. 1955 Dec;217(2):867–874. [PubMed] [Google Scholar]
- Rodgers J. B., Riley E. M., Drummey G. D., Isselbacher K. J. Lipid absorption in adrenalectomized rats: the role of altered enzyme activity in the intestinal mucosa. Gastroenterology. 1967 Oct;53(4):547–556. [PubMed] [Google Scholar]
- Segal S., Smith I. Delineation of cystine and cysteine transport systems in rat kidney cortex by developmental patterns. Proc Natl Acad Sci U S A. 1969 Jul;63(3):926–933. doi: 10.1073/pnas.63.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava L. M., Hübscher G. The effect of age on glycolytic and hexokinase activities in the mucosa of rat small intestine. Biochem J. 1968 Dec;110(3):607–608. doi: 10.1042/bj1100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States B., Segal S. Developmental aspects of cystine transport in rat intestinal segments. Biochim Biophys Acta. 1968 Sep 17;163(2):154–162. doi: 10.1016/0005-2736(68)90093-x. [DOI] [PubMed] [Google Scholar]
- States B., Segal S. Distribution of glutathione-cystine transhydrogenase activity in subcellular fractions of rat intestinal mucosa. Biochem J. 1969 Jun;113(2):443–444. doi: 10.1042/bj1130443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States B., Segal S. Thin-layer chromatographic separation of cystine and the N-ethylmaleimide adducts of cysteine and glutathionen. Anal Biochem. 1969 Feb;27(2):323–329. doi: 10.1016/0003-2697(69)90041-4. [DOI] [PubMed] [Google Scholar]
- Tietze F. Disulfide reduction in rat liver. II. Chromatographic separation of nucleotide-dependent disulfide reductase and GSH-disulfide transhydrogenase activities of the high-speed supernatant fraction. Biochim Biophys Acta. 1970 Dec 16;220(3):449–462. doi: 10.1016/0005-2744(70)90276-7. [DOI] [PubMed] [Google Scholar]
- Wendell P. L. Distribution of glutathione reductase and detection of glutathione-cystine transhydrogenase in rat tissues. Biochim Biophys Acta. 1968 Apr 24;159(1):179–181. doi: 10.1016/0005-2744(68)90257-x. [DOI] [PubMed] [Google Scholar]


