Abstract
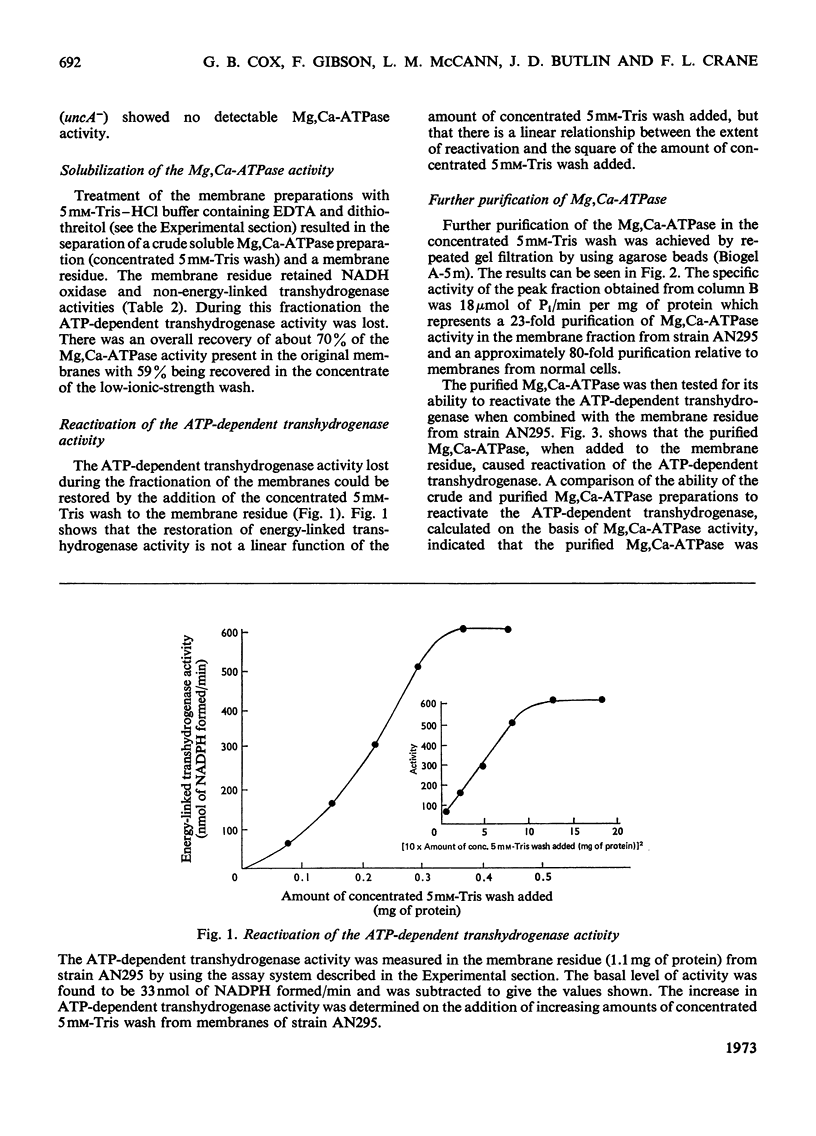
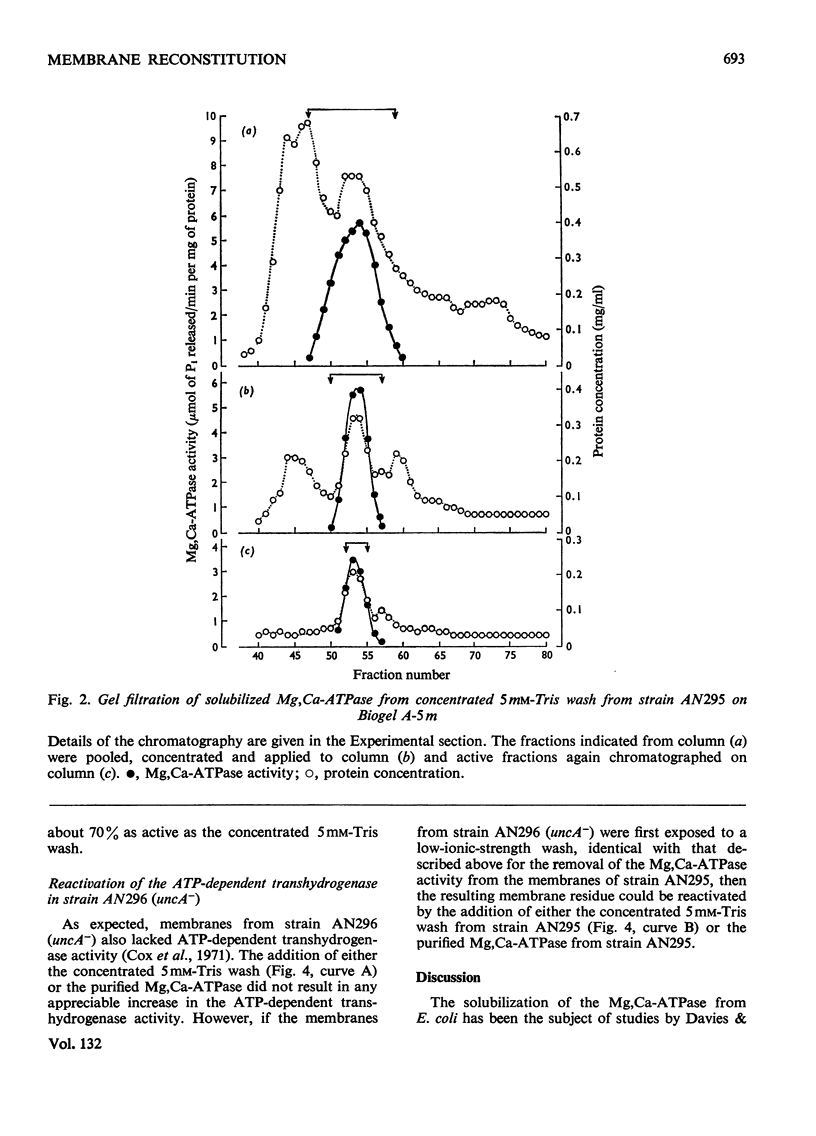
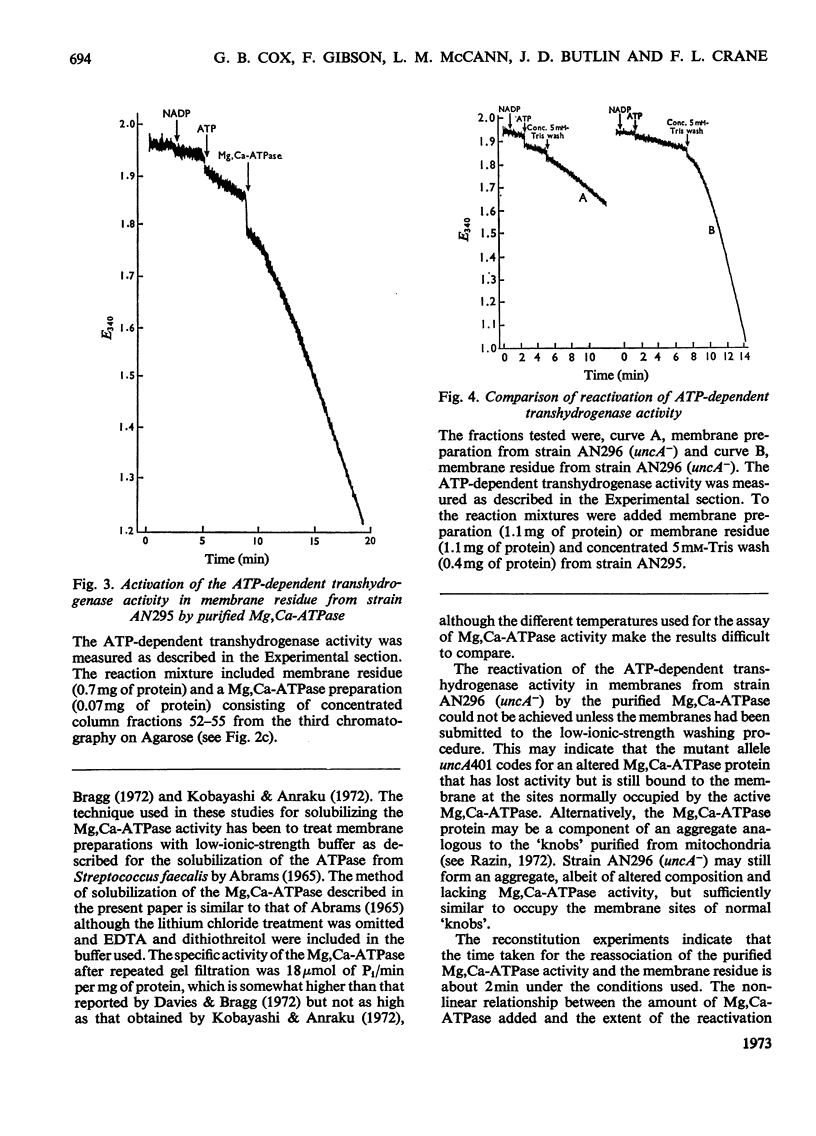
1. We have isolated a mutant of Escherichia coli K12 (strain AN295) that forms de-repressed amounts of Mg2+,Ca2+-stimulated adenosine triphosphatase. 2. The Mg2+,Ca2+-stimulated triphosphatase activity was separated from membrane preparations from strain AN295 by extraction with 5mm-Tris–HCl buffer containing EDTA and dithiothreitol, resulting in a loss of the ATP-dependent transhydrogenase activity. The non-energy-linked transhydrogenase activity remained in the membrane residue. 3. The solubilized Mg2+,Ca2+-stimulated adenosine triphosphatase activity from strain AN295 was partially purified by repeated gel filtration. The addition of the purified Mg2+,Ca2+-stimulated adenosine triphosphatase to the membrane residue from strain AN295 reactivated the ATP-dependent transhydrogenase activity. 4. Strain AN296, lacking Mg2+,Ca2+-stimulated adenosine triphosphatase activity, was derived by transducing the mutant allele, uncA401, into strain AN295. The ATP-dependent transhydrogenase activity was lost but the non-energy linked transhydrogenase was retained. 5. The ATP-dependent transhydrogenase activity in membrane preparations from strain AN296 (uncA−) could not be re-activated by the purified Mg2+,Ca2+-stimulated adenosine triphosphatase from strain AN295. However, after extraction by 5mm-Tris–HCl buffer containing EDTA and dithiothreitol, the ATP-dependent transhydrogenase activity could be re-activated by the addition of the purified Mg2+,Ca2+-stimulated adenosine triphosphatase from strain AN295 to the membrane residue from strain AN296 (uncA−).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- Ball E. G., Cooper O. THE OXIDATION OF REDUCED TRIPHOSPHOPYRIDINE NUCLEOTIDE AS MEDIATED BY THE TRANSHYDROGENASE REACTION AND ITS INHIBITION BY THYROXINE. Proc Natl Acad Sci U S A. 1957 May 15;43(5):357–364. doi: 10.1073/pnas.43.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C., Abrams A. Isolation of a bacterial membrane protein, nectin, essential for the attachment of adenosine triphosphatase. J Biol Chem. 1971 Mar 10;246(5):1542–1544. [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., Pittard J. Mutant strains of Escherichia coli K-12 unable to form ubiquinone. J Bacteriol. 1968 May;95(5):1591–1598. doi: 10.1128/jb.95.5.1591-1598.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Butlin J. D., Gibson F. The energy-linked transhydrogenase reaction in respiratory mutants of Escherichia coli K12. Biochem J. 1971 Nov;125(2):489–493. doi: 10.1042/bj1250489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. L., Bragg P. D. Properties of a soluble Ca 2+ - and Mg 2+ -activated ATPase released from Escherichia coli membranes. Biochim Biophys Acta. 1972 Apr 14;266(1):273–284. doi: 10.1016/0005-2736(72)90142-3. [DOI] [PubMed] [Google Scholar]
- Kagawa Y. Reconstitution of oxidative phosphorylation. Biochim Biophys Acta. 1972 Aug 4;265(3):297–338. [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Anraku Y. Membrane-bound adenosine triphosphatase of Escherichia coli. I. Partial purification and properties. J Biochem. 1972 Mar;71(3):387–399. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- PITTARD J. EFFECT OF INTEGRATED SEX FACTOR ON TRANSDUCTION OF CHROMOSOMAL GENES IN ESCHERICHIA COLI. J Bacteriol. 1965 Mar;89:680–686. doi: 10.1128/jb.89.3.680-686.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. Reconstruction of biological membranes. Biochim Biophys Acta. 1972 Apr 18;265(2):241–296. [PubMed] [Google Scholar]
- STEIN A. M., KAPLAN N. O., CIOTTI M. M. Pyridine nucleotide transhydrogenase. VII. Determination of the reactions with coenzyme analogues in mammalian tissues. J Biol Chem. 1959 Apr;234(4):979–986. [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


