Abstract
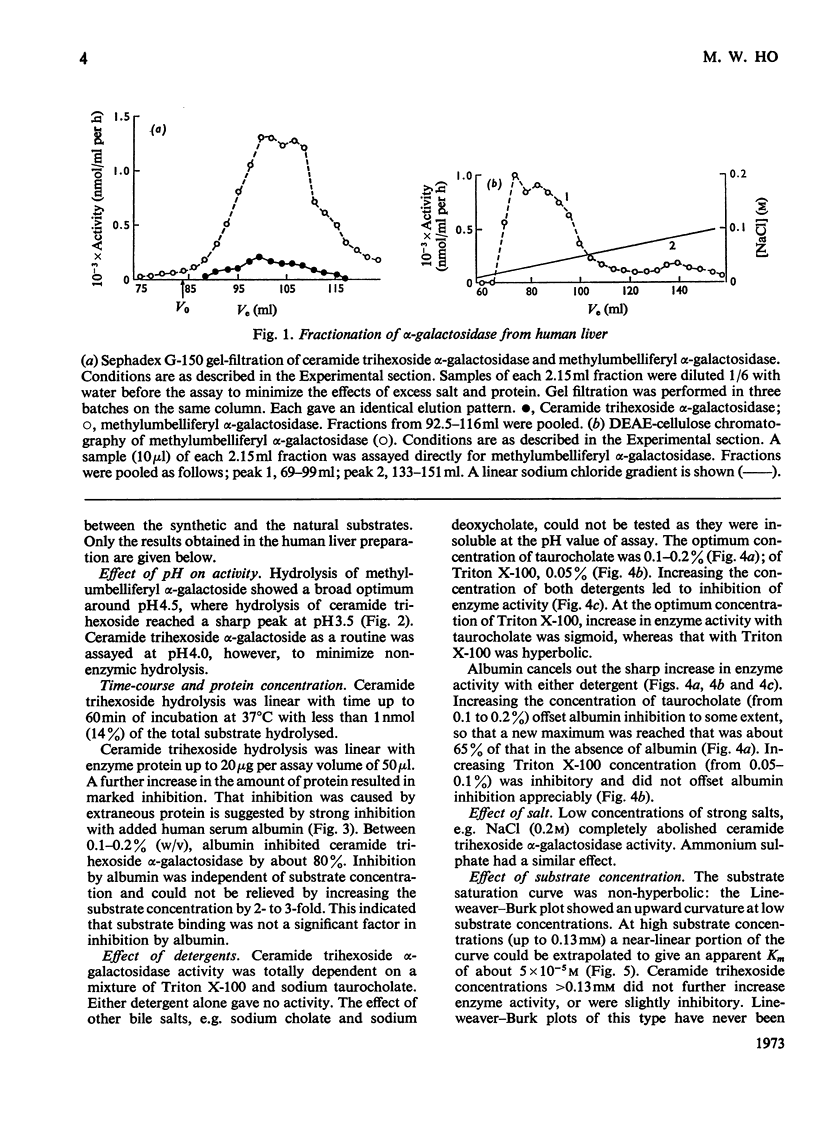
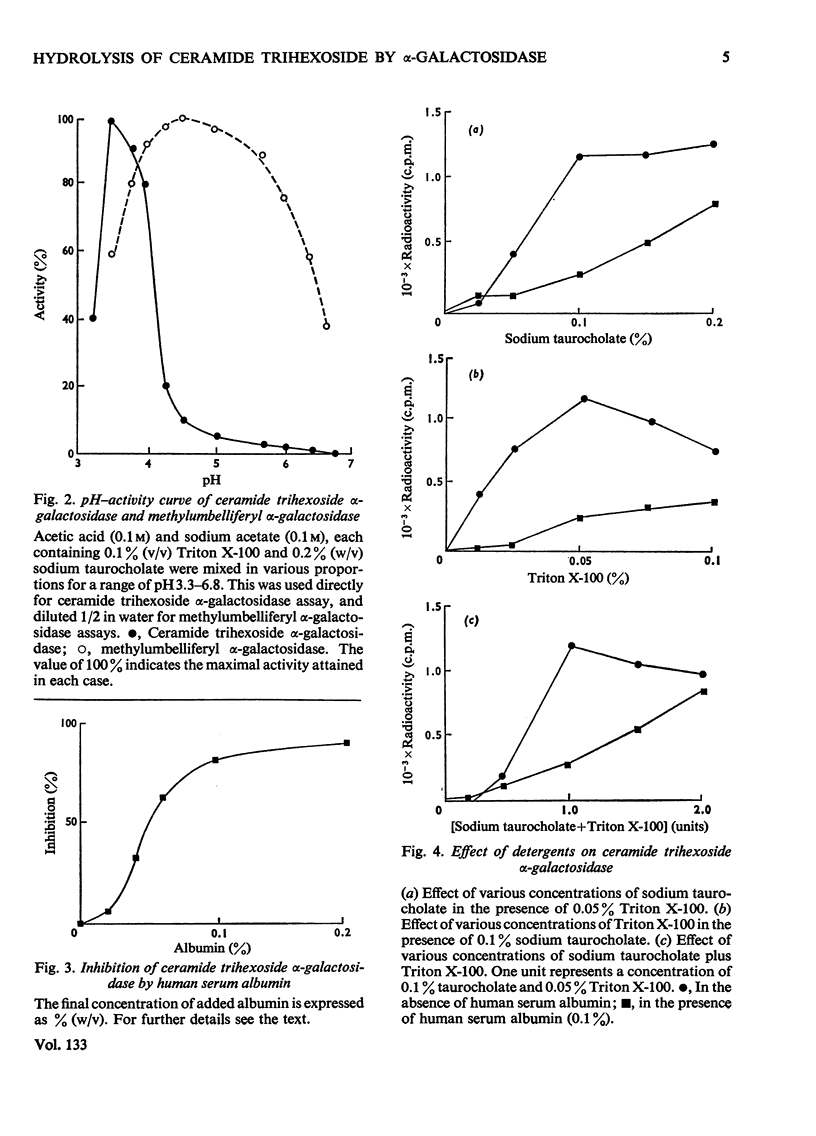
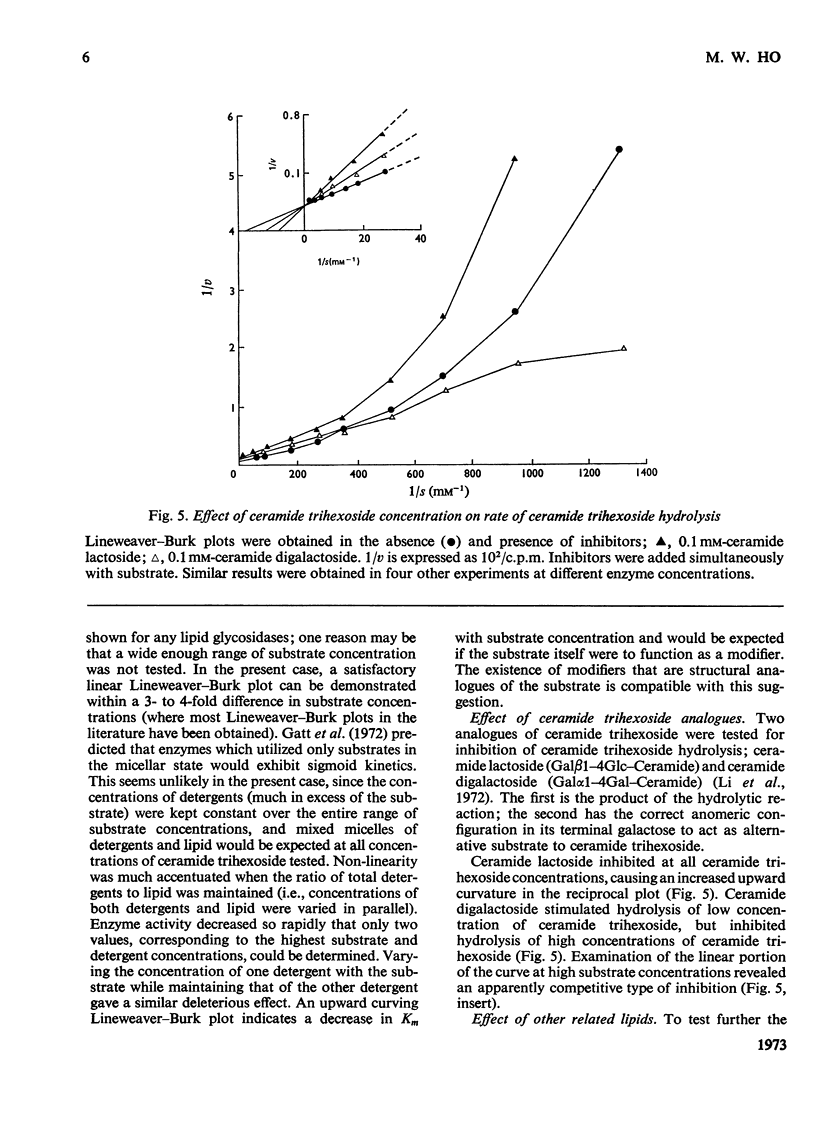
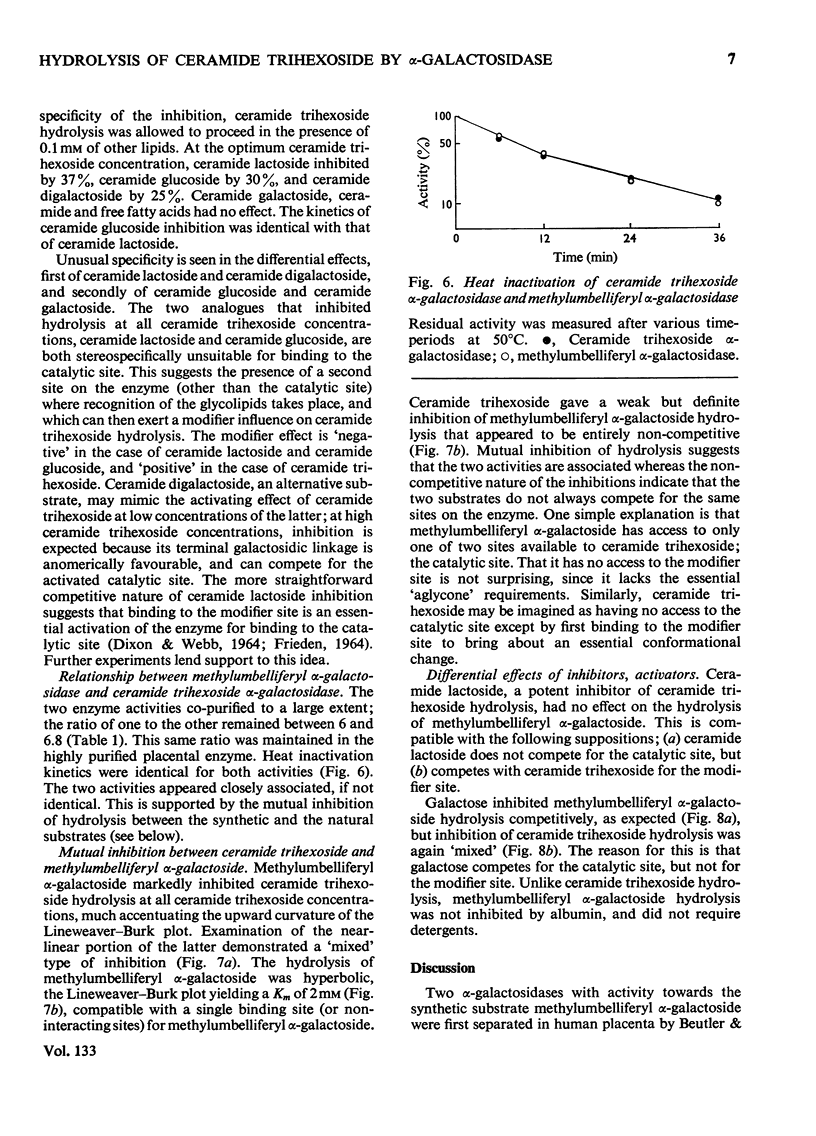
1. Partially purified ceramide trihexoside α-galactosidase from human liver was studied by using ceramide trihexoside specifically tritiated in the terminal galactose. 2. The hydrolysis of ceramide trihexoside was absolutely dependent on a mixture of sodium taurocholate and Triton X-100 and was markedly inhibited by human serum albumin and by NaCl. 3. The Lineweaver–Burk plot for ceramide trihexoside hydrolysis was upward curving. Ceramide lactoside inhibited hydrolysis of all concentrations of ceramide trihexoside. Ceramide digalactoside stimulated hydrolysis of low concentrations of ceramide trihexoside, but inhibited hydrolysis of high concentrations of the lipid. 4. α-Galactosidase activity assayed with the synthetic substrate 4-methylumbelliferyl α-d-galactopyranoside fractionated together with activity assayed with the natural substrate ceramide trihexoside. Both activities had identical heat-inactivation kinetics. 5. Characteristics of the hydrolysis of the synthetic substrate differed considerably from those of the natural substrate, including pH optimum, shape of the Lineweaver–Burk plot, and differential effects of inhibitors and activators. Mutual inhibition of hydrolysis between the synthetic and natural substrates was predominantly non-competitive. 6. These results are discussed in the light of special problems involved in the hydrolysis of lipids in an aqueous milieu.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arora R. C., Radin N. S. Ceramide-like synthetic amides that inhibit cerebroside galactosidase. J Lipid Res. 1972 Jan;13(1):86–91. [PubMed] [Google Scholar]
- Bensaude I., Callahan J., Philippart M. Fabry's disease as an -galactosidosis: evidence for an -configuration in trihexosyl ceramide. Biochem Biophys Res Commun. 1971 May 21;43(4):913–918. doi: 10.1016/0006-291x(71)90704-2. [DOI] [PubMed] [Google Scholar]
- Beutler E., Kuhl W. Relationship between human alpha-galactosidase isozymes. Nat New Biol. 1972 Oct 18;239(94):207–208. doi: 10.1038/newbio239207b0. [DOI] [PubMed] [Google Scholar]
- Bowen D. M., Radin N. S. Properties of cerebroside galactosidase. Biochim Biophys Acta. 1968 May 1;152(3):599–610. doi: 10.1016/0005-2760(68)90100-8. [DOI] [PubMed] [Google Scholar]
- Brady R. O., Gal A. E., Bradley R. M., Mårtensson E. The metabolism of ceramide trihexosides. I. Purification and properties of an enzyme that cleaves the terminal galactose molecule of galactosylgalactosylglucosylceramide. J Biol Chem. 1967 Mar 10;242(5):1021–1026. [PubMed] [Google Scholar]
- Cherry R. J., Berger K. U., Chapman D. Interaction of erythrocyte apoprotein with bimolecular lipid membranes. Biochem Biophys Res Commun. 1971 Aug 6;44(3):644–652. doi: 10.1016/s0006-291x(71)80132-8. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FRIEDEN C. TREATMENT OF ENZYME KINETIC DATA. I. THE EFFECT OF MODIFIERS ON THE KINETIC PARAMETERS OF SINGLE SUBSTRATE ENZYMERS. J Biol Chem. 1964 Oct;239:3522–3531. [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gatt S. Enzymic hydrolysis of sphingolipids: Hydrolysis of ceramide glucoside by an enzyme from ox brain. Biochem J. 1966 Dec;101(3):687–691. [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. I., Siddiqui B., Li Y. T., Li S. C., Hellerqvist C. G. Anomeric structure of globoside and ceramide grihexoside of human erythrocytes and hamster fibroblasts. J Biol Chem. 1971 Apr 10;246(7):2271–2277. [PubMed] [Google Scholar]
- Ho M. W., Beutler S., Tennant L., O'Brien J. S. Fabry's disease: evidence for a physically altered -galactosidase. Am J Hum Genet. 1972 May;24(3):256–266. [PMC free article] [PubMed] [Google Scholar]
- Li Y. T., Li S. C., Dawson G. Anomeric structure of ceramide digalactoside isolated from the kidney of a patient with Fabry's disease. Biochim Biophys Acta. 1972 Jan 27;260(1):88–92. [PubMed] [Google Scholar]
- Mapes C. A., Anderson R. L., Sweeley C. C. Galactosylgalactosylglucosylceramide: Galactosyl hydrolase in normal human plasma and its absence in patients with fabry's disease. FEBS Lett. 1970 Apr 2;7(2):180–182. doi: 10.1016/0014-5793(70)80151-x. [DOI] [PubMed] [Google Scholar]
- Morell P., Braun P. Biosynthesis and metabolic degradation of sphingolipids not containing sialic acid. J Lipid Res. 1972 May;13(3):293–310. [PubMed] [Google Scholar]
- Parsons J. G., Patton S. Two-dimensional thin-layer chromatography of polar lipids from milk and mammary tissue. J Lipid Res. 1967 Nov;8(6):696–698. [PubMed] [Google Scholar]
- Radin N. S., Hof L., Bradley R. M., Brady R. O. Lactosylceramide galactosidase: comparison with other sphingolipid hydrolases in developing rat brain. Brain Res. 1969 Jul;14(2):497–505. doi: 10.1016/0006-8993(69)90124-3. [DOI] [PubMed] [Google Scholar]
- Razin S. Reconstruction of biological membranes. Biochim Biophys Acta. 1972 Apr 18;265(2):241–296. [PubMed] [Google Scholar]
- Sweet C., Zull J. E. The binding of serum albumin to phospholipid liposomes. Biochim Biophys Acta. 1970 Dec 1;219(2):253–262. doi: 10.1016/0005-2736(70)90204-x. [DOI] [PubMed] [Google Scholar]
- Wenger D. A., Wardell S. Action of neuraminidase from Clostridium perfringens of Tay-Sachs ganglioside. Physiol Chem Phys. 1972;4(3):224–230. [PubMed] [Google Scholar]


