Abstract
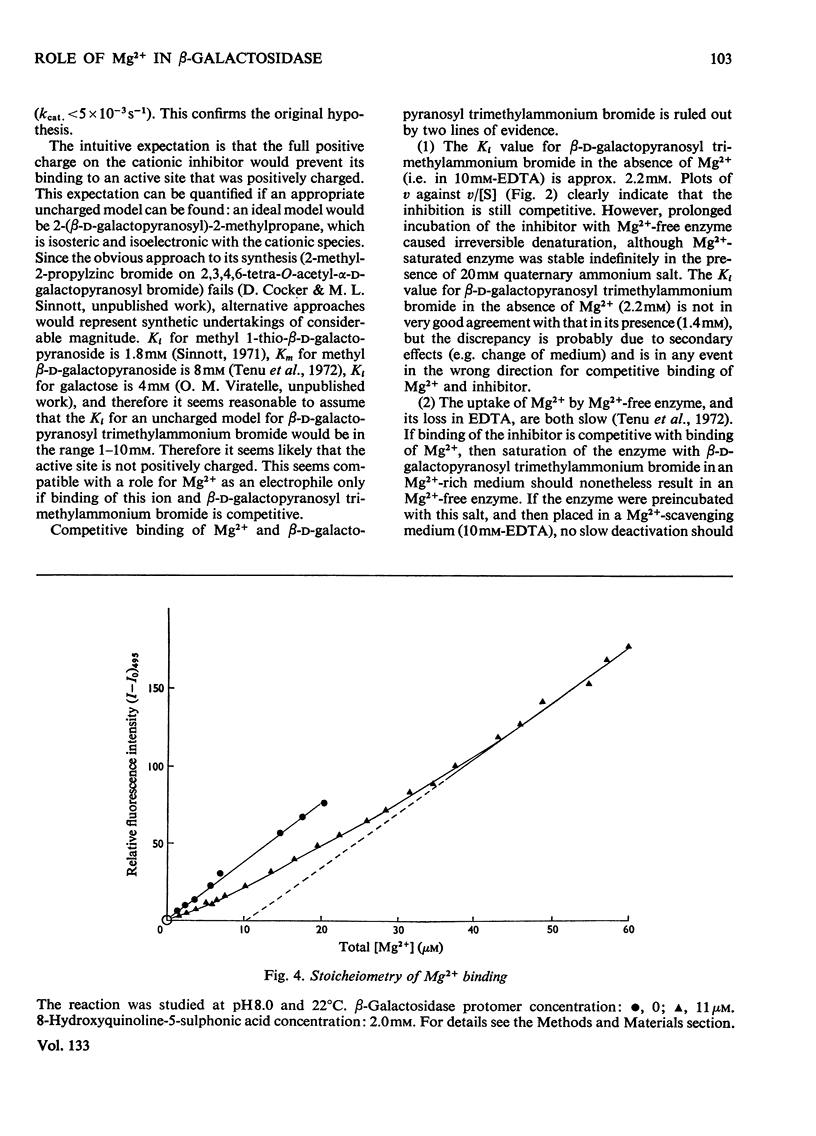
1. β-d-Galactopyranosyl trimethylammonium bromide is a competitive inhibitor of β-galactosidase, Ki=1.4±0.2mm at 25°C. 2. Tetramethylammonium bromide is not an inhibitor (Ki>0.2m). 3. The kinetics of deactivation of Mg2+-saturated, and of inhibitor-and Mg2+-saturated, enzyme in 10mm-EDTA are similar. 4. The apparent Ki for the glycosylammonium salt is approx. 2.2mm in the absence of Mg2+. 5. It is therefore concluded that Mg2+ and the inhibitor bind independently, and that the Mg2+ does not act as an electrophilic catalyst. 6. Complexant fluorescence measurements indicate binding of 1 Mg2+ ion per 135000-dalton protomer. 7. This stoicheiometry is confirmed by equilibrium dialysis. 8. 1,6-Anhydrogalactopyranose is neither a substrate (kcat./Km< 3×10−2m−1·S−1) nor an inhibitor (Ki>0.2m). 9. Considerations of conformations available to the cationic inhibitor and to the anhydrogalactose indicate that the substrate is bound with the pyranose ring in a conformation not greatly different from the normal chair (C1) conformation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chipman D. M., Sharon N. Mechanism of lysozyme action. Science. 1969 Aug 1;165(3892):454–465. doi: 10.1126/science.165.3892.454. [DOI] [PubMed] [Google Scholar]
- Halford S. E., Schlesinger M. J., Gutfreund H. Escherichia coli alkaline phosphatase. Kinetic studies with the tetrameric enzyme. Biochem J. 1972 Mar;126(5):1081–1090. doi: 10.1042/bj1261081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott M. L. -galactosidase-catalysed hydrolysis of -D-galactopyranosyl azide. Biochem J. 1971 Dec;125(3):717–719. doi: 10.1042/bj1250717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott M. L., Souchard I. J. The mechanism of action of beta-galactosidase. Effect of aglycone nature and -deuterium substitution on the hydrolysis of aryl galactosides. Biochem J. 1973 May;133(1):89–98. doi: 10.1042/bj1330089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenu J. P., Viratelle O. M., Garnier J., Yon J. pH dependence of the activity of beta-galactosidase from Escherichia coli. Eur J Biochem. 1971 Jun 11;20(3):363–370. doi: 10.1111/j.1432-1033.1971.tb01402.x. [DOI] [PubMed] [Google Scholar]
- Tenu J. P., Viratelle O. M., Yon J. Kinetic study of the activation process of -galactosidase from Escherichia coli by Mg 2+ . Eur J Biochem. 1972 Mar 15;26(1):112–118. doi: 10.1111/j.1432-1033.1972.tb01746.x. [DOI] [PubMed] [Google Scholar]
- WALLENFELS K., MALHOTRA O. P. Galactosidases. Adv Carbohydr Chem. 1961;16:239–298. doi: 10.1016/s0096-5332(08)60264-7. [DOI] [PubMed] [Google Scholar]


