Abstract
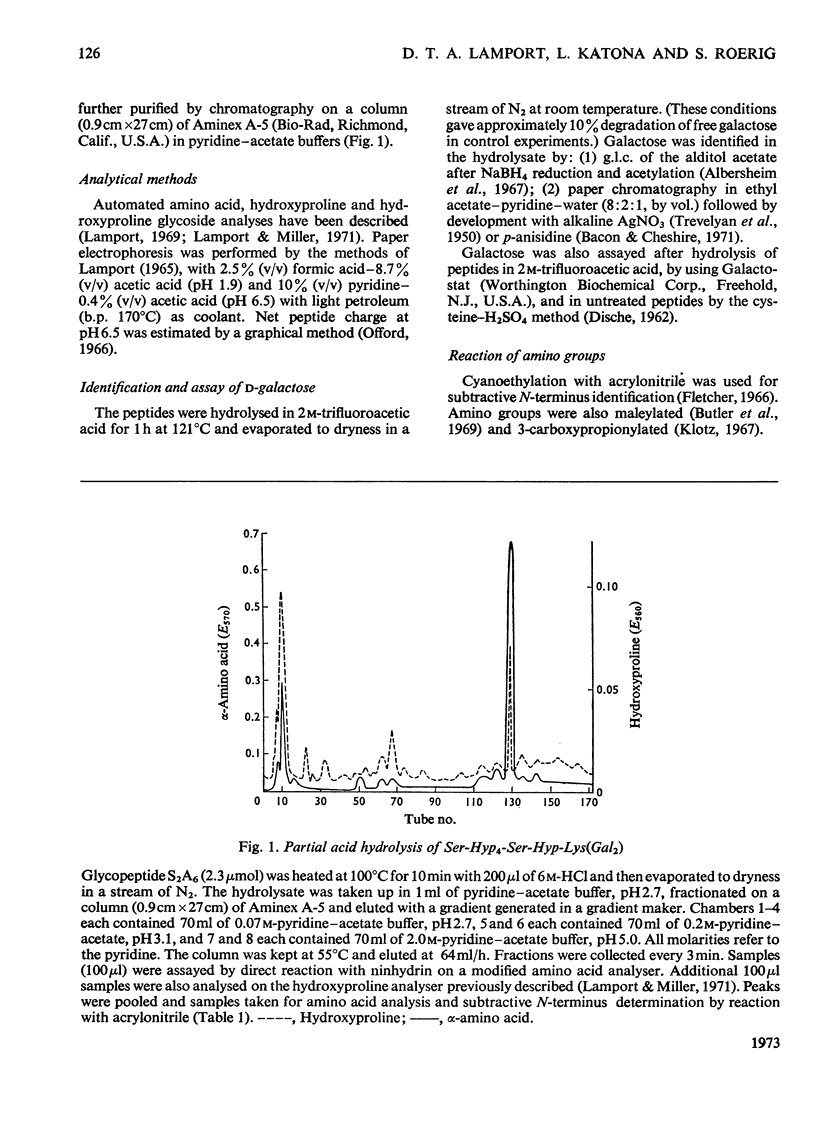
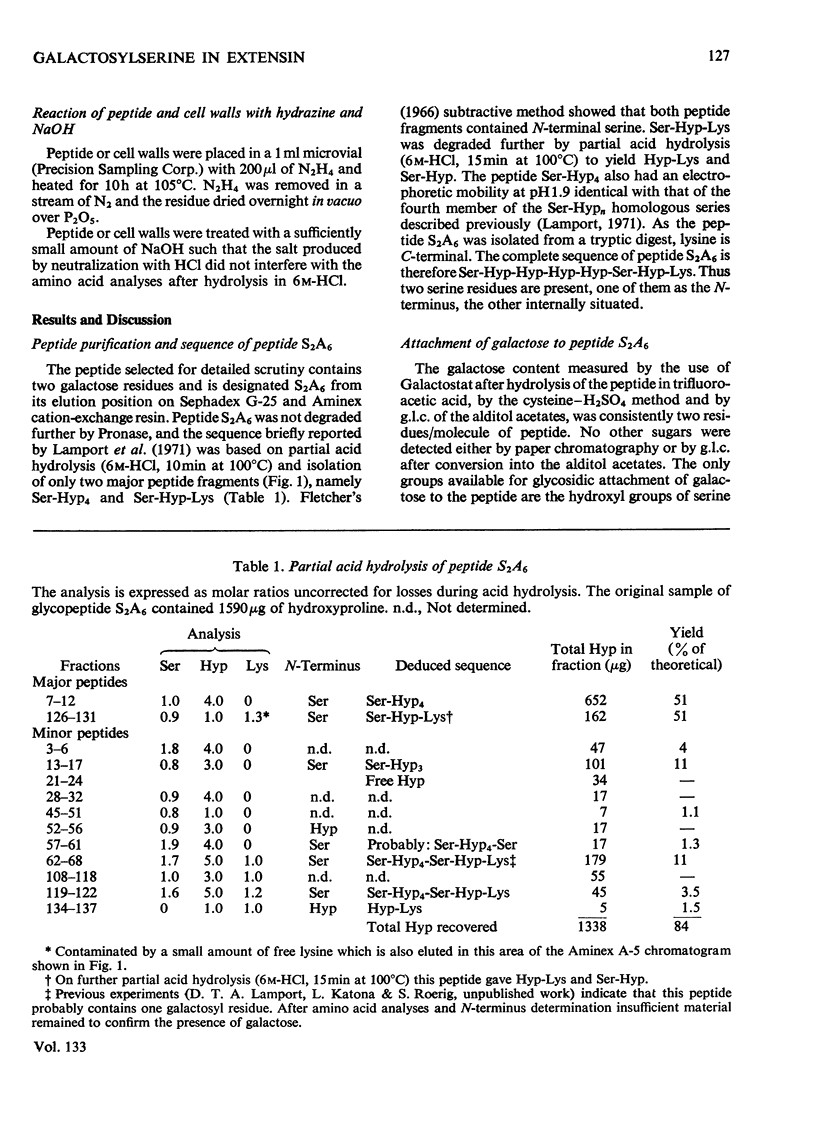
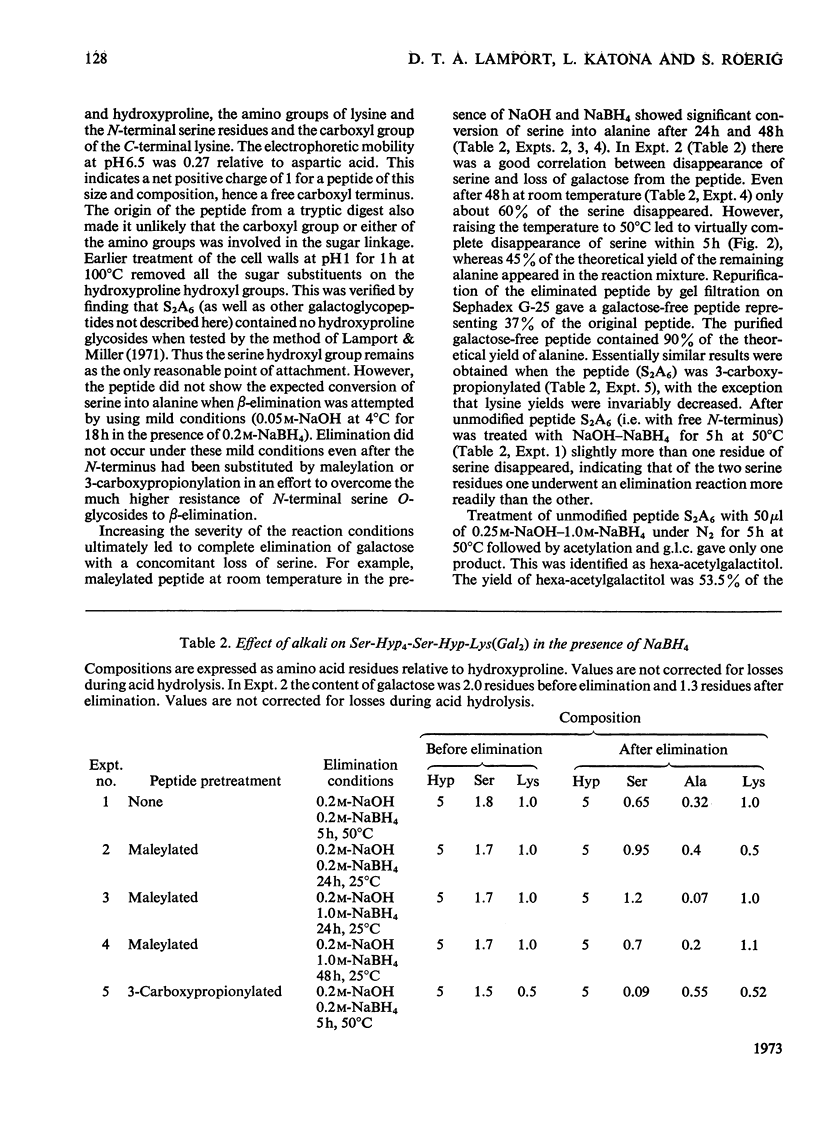
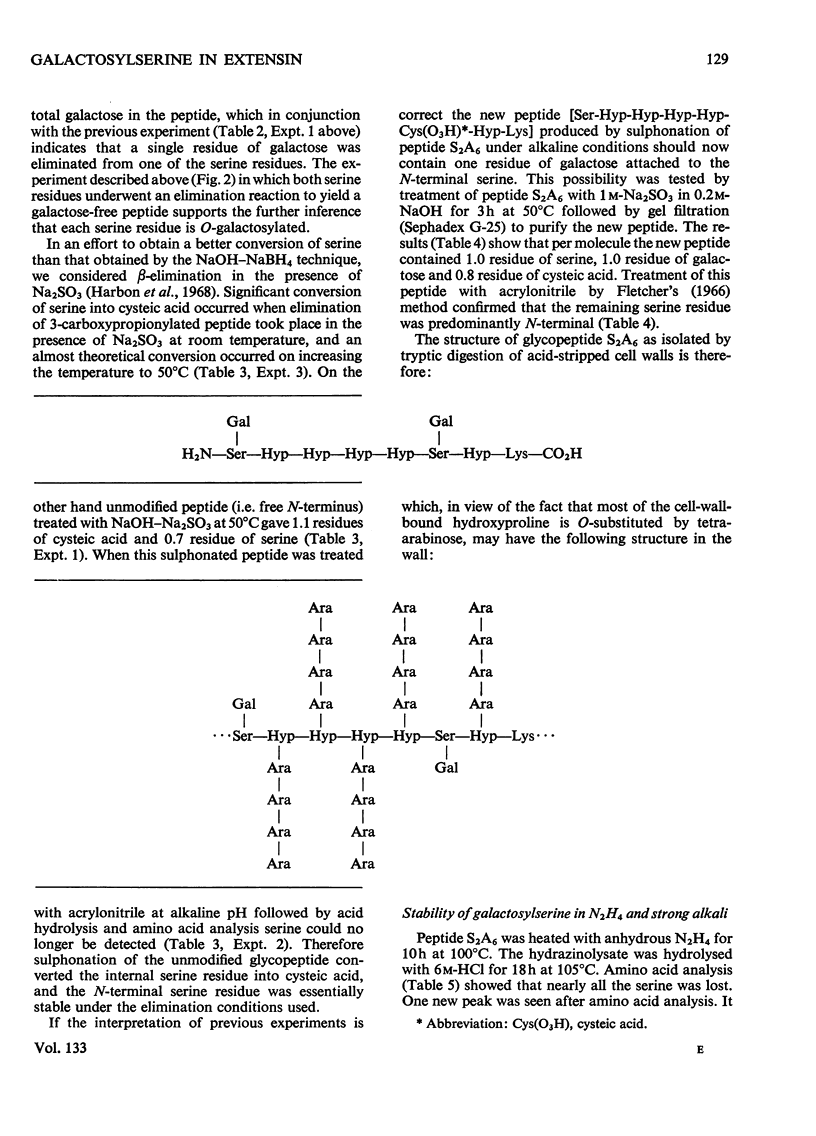
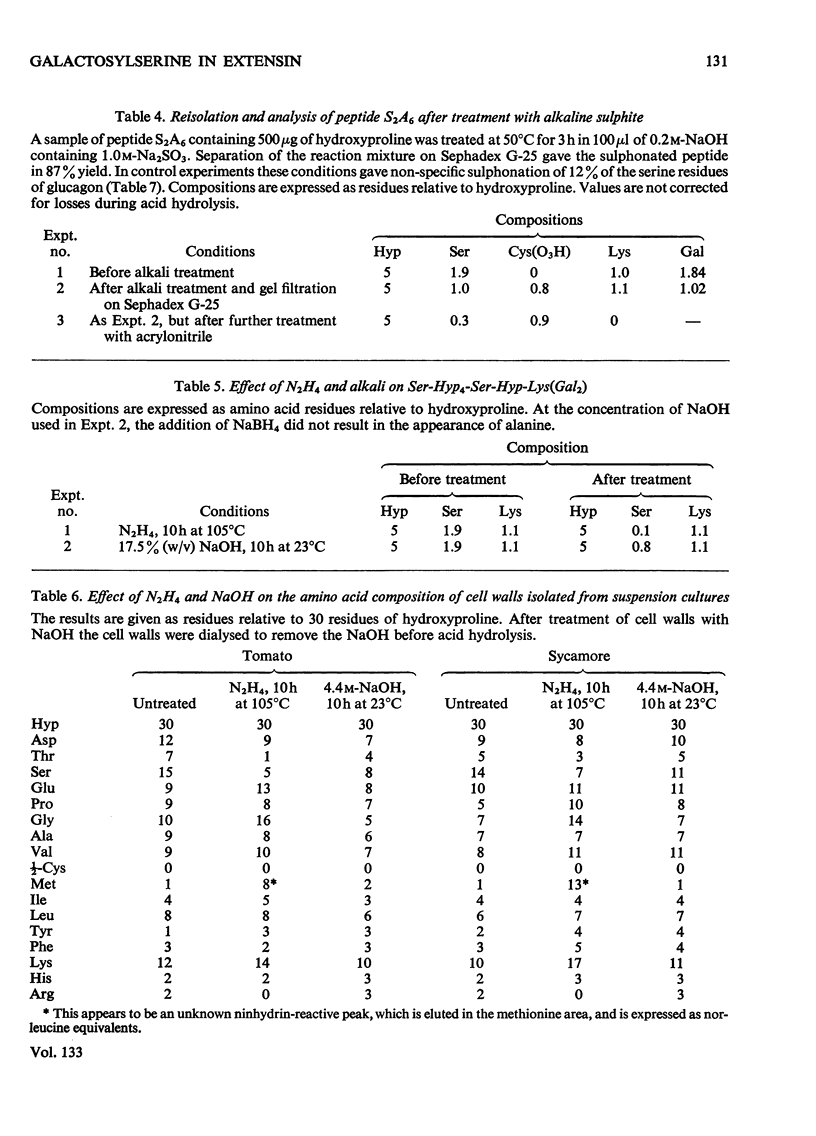
Cell walls obtained from tomato suspension cultures were treated at pH1 for 1h at 100°C to remove arabinose oligosaccharide substituents from the hydroxyproline residues of extensin. Tryptic attack of these acid-stripped walls yielded glycopeptides containing galactose. When one of these glycopeptides (designated S2A6; sequence NH2-Ser-Hyp-Hyp-Hyp-Hyp-Ser-Hyp-Lys-CO2H) was treated with (a) NaOH–NaBH4 or (b) NaOH–Na2SO3 some of the serine was converted into (a) alanine or (b) cysteic acid, and the peptide lost galactose. Maleylation or 3-carboxypropionylation of N-terminal serine was necessary for conversion of this residue and for complete loss of galactose. These results indicate that a single galactose residue is attached O-glycosidically to each of the two serine residues. Hydrazinolysis of peptide S2A6 or of isolated cell walls also led to destruction of serine. In control experiments non-glycosylated serine was not destroyed during hydrazinolysis. Thus the galactosylserine linkage is sensitive to N2H4.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon J. S., Cheshire M. V. Apiose and mono-O-methyl sugars as minor constituents of the leaves of deciduous trees and various other species. Biochem J. 1971 Sep;124(3):555–562. doi: 10.1042/bj1240555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P. J., Harris J. I., Hartley B. S., Lebeman R. The use of maleic anhydride for the reversible blocking of amino groups in polypeptide chains. Biochem J. 1969 May;112(5):679–689. doi: 10.1042/bj1120679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbon S., Herman G., Clauser S. Quantitative evaluation of O-glycosidic linkages between sugars and aminoacids in ovine submaxillary gland mucoprotein. Eur J Biochem. 1968 Apr 3;4(2):265–272. doi: 10.1111/j.1432-1033.1968.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Heath M. F., Northcote D. H. Glycoprotein of the wall of sycamore tissue-culture cells. Biochem J. 1971 Dec;125(4):953–961. doi: 10.1042/bj1250953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPORT D. T. CELL SUSPENSION CULTURES OF HIGHER PLANTS: ISOLATION AND GROWTH ENERGETICS. Exp Cell Res. 1964 Jan;33:195–206. doi: 10.1016/s0014-4827(64)81026-0. [DOI] [PubMed] [Google Scholar]
- Lamport D. T., Miller D. H. Hydroxyproline arabinosides in the plant kingdom. Plant Physiol. 1971 Oct;48(4):454–456. doi: 10.1104/pp.48.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport D. T. The isolation and partial characterization of hydroxyproline-rich glycopeptides obtained by enzymic degradation of primary cell walls. Biochemistry. 1969 Mar;8(3):1155–1163. doi: 10.1021/bi00831a049. [DOI] [PubMed] [Google Scholar]
- Miller D. H., Lamport D. T., Miller M. Hydroxyproline heterooligosaccharides in Chlamydomonas. Science. 1972 May 26;176(4037):918–920. doi: 10.1126/science.176.4037.918. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]


