Abstract
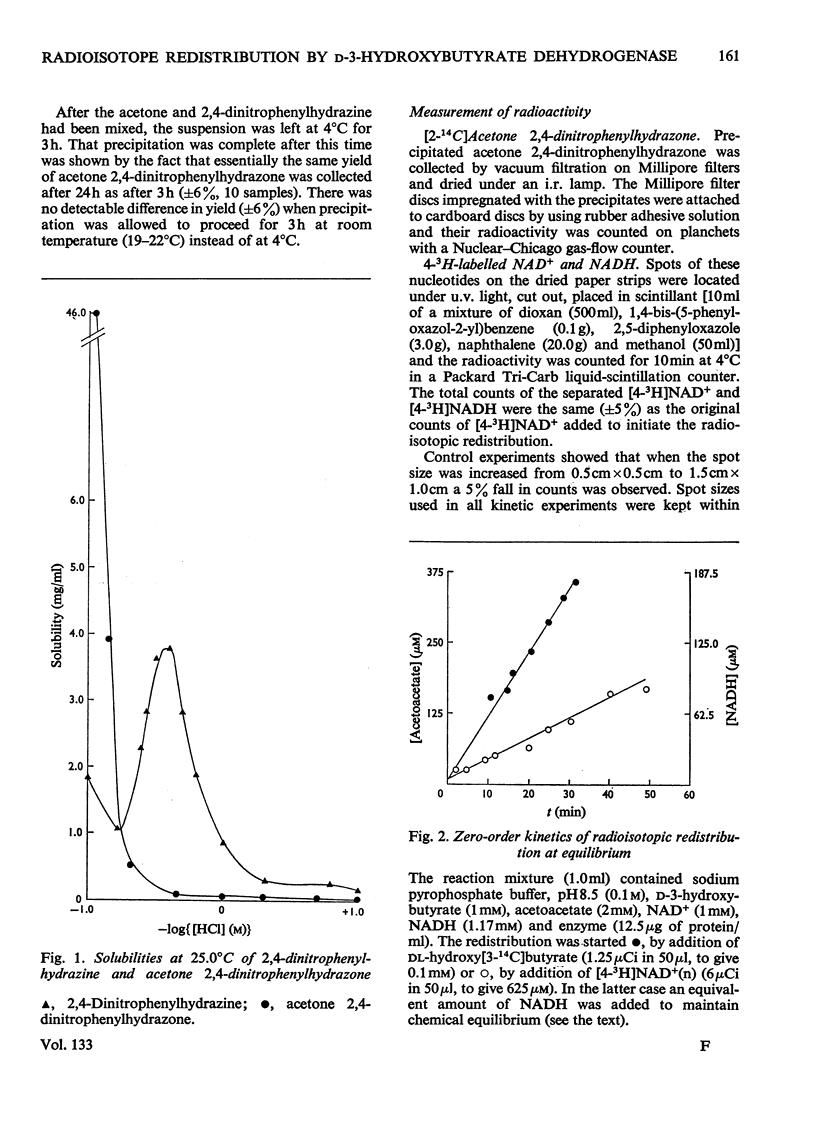
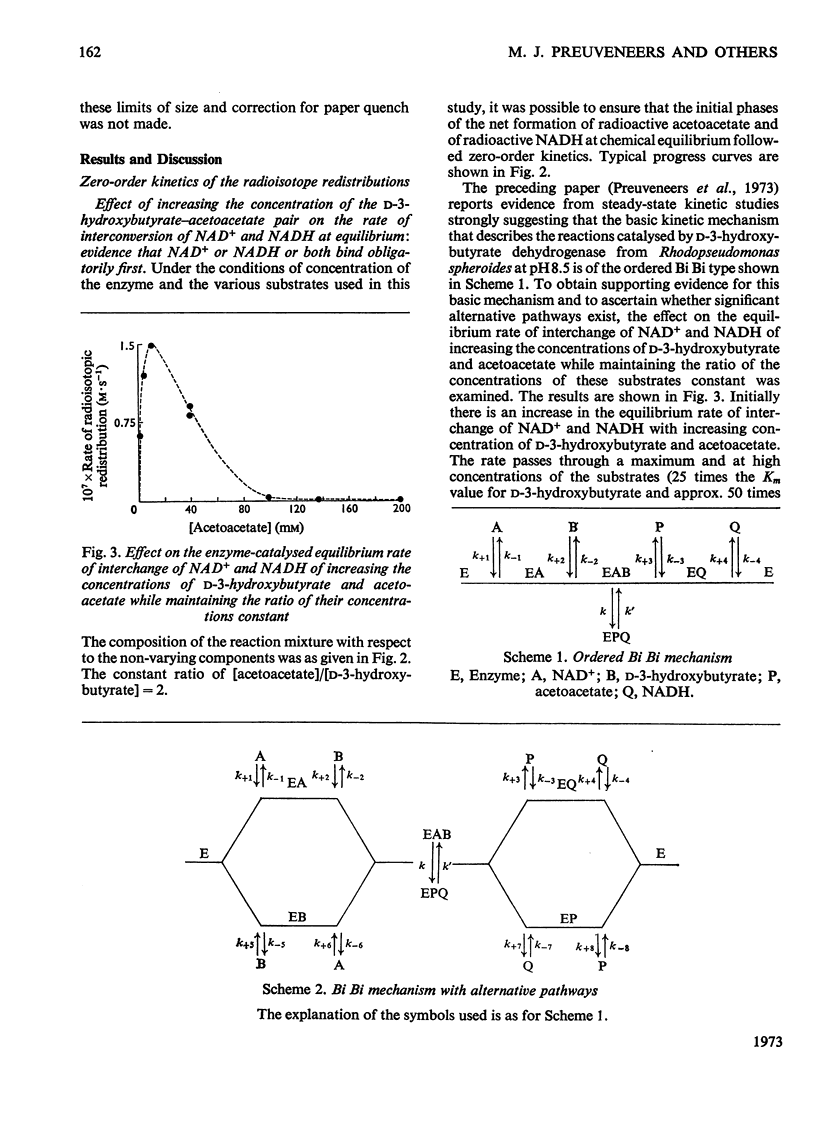
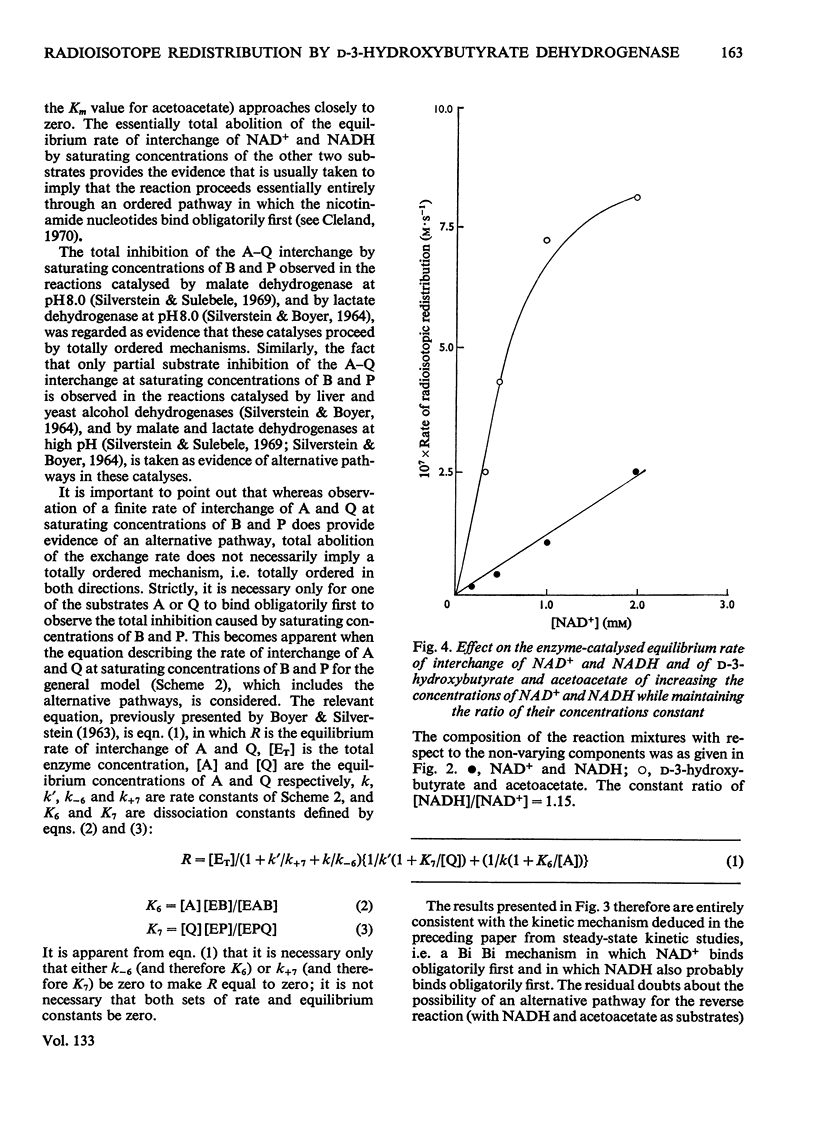
1. The reversible NAD+-linked oxidation of d-3-hydroxybutyrate to acetoacetate in 0.1m-sodium pyrophosphate buffer, pH8.5, at 25.0°C, catalysed by d-3-hydroxybutyrate dehydrogenase (d-3-hydroxybutyrate–NAD+ oxidoreductase, EC 1.1.1.30), was studied kinetically at chemical equilibrium by monitoring radioisotope redistribution with sodium dl-hydroxy[3-14C]butyrate and [4-3H]NAD+(labelled in the nicotinamide ring). 2. When all substrates are maintained at concentrations approaching saturation (approx. 3–50 times the Km values) the first-order rate constant for the enzyme-catalysed interconversion of NAD+ and NADH is much smaller than that for the enzyme-catalysed interconversion of d-3-hydroxybutyrate and acetoacetate. 3. The rate of interconversion of NAD+ and NADH increases initially with increasing concentrations of d-3-hydroxybutyrate and acetoacetate (ratio of concentrations maintained constant), passes through a maximum and approaches closely to zero at saturating concentrations of the latter substrates. 4. The rates of interconversion of NAD+ and NADH and of d-3-hydroxybutyrate and acetoacetate increase with increasing concentration of NAD+ (up to 66 times its Km value) and NADH (up to 180 times its Km value) (ratio of the concentrations of the nicotinamide nucleotides maintained constant). 5. These findings support the description of this catalysis as an ordered Bi Bi mechanism with no detectable alternative pathway, in which the interconversion of the central ternary complexes is not rate-limiting, and provide no evidence for the formation of dead-end complexes. 6. The solubility of 2,4-dinitrophenylhydrazine in HCl exhibits an acidity optimum, the maximum solubility at 25.0°C (3.8mg/ml, 19mm) occurring at 2.29m-HCl; in solutions of this acidity acetone 2,4-dinitrophenylhydrazone is relatively insoluble (0.098mg/ml, 0.413mm).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYER P. D. Uses and limitations of measurements of rates of isotopic exchange and incorporation in catalyzed reactions. Arch Biochem Biophys. 1959 Jun;82(2):387–410. doi: 10.1016/0003-9861(59)90136-5. [DOI] [PubMed] [Google Scholar]
- Balinsky D., Dennis A. W., Cleland W. W. Kinetic and isotope-exchange studies on shikimate dehydrogenase from Pisum sativum. Biochemistry. 1971 May 11;10(10):1947–1952. doi: 10.1021/bi00786a032. [DOI] [PubMed] [Google Scholar]
- Britton H. G. The chemical estimation of ketone bodies. Anal Biochem. 1966 May;15(2):261–269. doi: 10.1016/0003-2697(66)90030-3. [DOI] [PubMed] [Google Scholar]
- Preuveneers M. J., Peacock D., Crook E. M., Clark J. B., Brocklehurst K. D-3-hydroxybutyrate dehydrogenase from Rhodopseudomonas spheroides. Kinetic mechanism from steady-state kinetics of the reaction catalysed by the enzyme in solution and covalently attached to diethylaminoethylcellulose. Biochem J. 1973 May;133(1):133–157. doi: 10.1042/bj1330133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERSTEIN E., BOYER P. D. EQUILIBRIUM REACTION RATES AND THE MECHANISMS OF BOVINE HEART AND RABBIT MUSCLE LACTATE DEHYDROGENASES. J Biol Chem. 1964 Nov;239:3901–3907. [PubMed] [Google Scholar]
- Silverstein E. Separation of NAD+ and NADH and NADP+ aned NADPH by anion exchange on DEAE-cellulose paper. Biochim Biophys Acta. 1970 Jul 21;215(1):205–206. doi: 10.1016/0304-4165(70)90409-5. [DOI] [PubMed] [Google Scholar]
- Silverstein E., Sulebele G. Catalytic mechanism of pig heart mitochondrial malate dehydrogenase studied by kinetics at equilibrium. Biochemistry. 1969 Jun;8(6):2543–2550. doi: 10.1021/bi00834a042. [DOI] [PubMed] [Google Scholar]


