Abstract
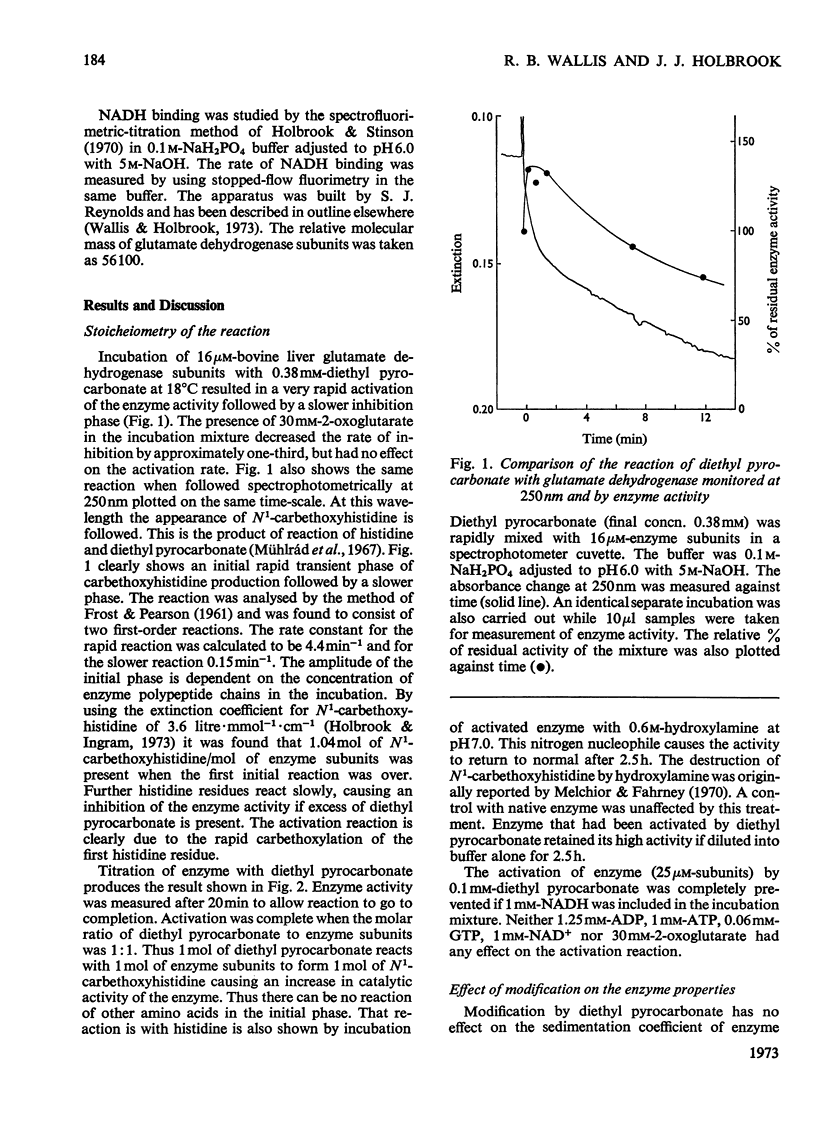
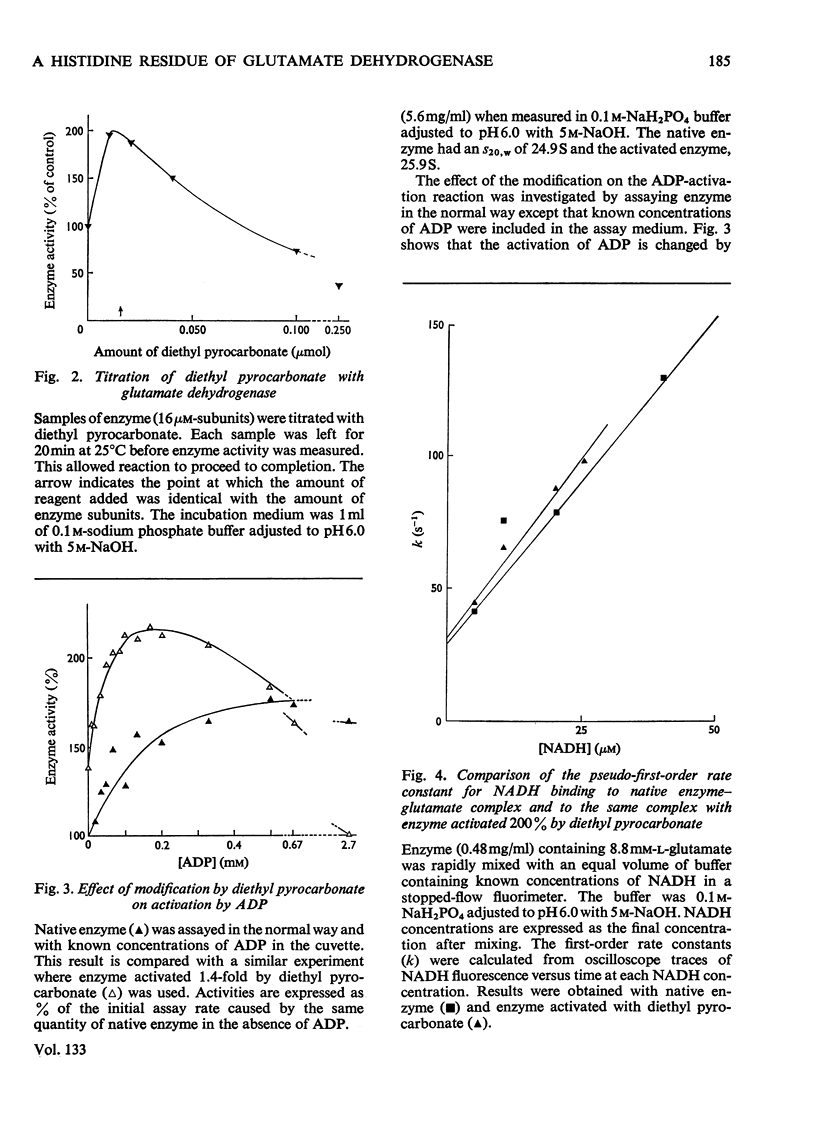
1. One mol of diethyl pyrocarbonate will react with one mol of glutamate dehydrogenase polypeptide chains to form one mol of N1-carbethoxyhistidine. Reaction is prevented by NADH. 2. The 1:1 complex has an increased specific activity (1.4–2.0-fold). 3. The reason for the activation is discussed. The results are not consistent with NADH dissociation from the enzyme–glutamate–NADH complex being rate-limiting in the steady state measured. 4. The effects of modification on the properties of the enzyme were investigated. The effects of GTP and NAD+ on the enzyme activity are unaltered by activation. NADH binding is unaltered and there is no apparent change in the molecular weight. However, the activated enzyme can still be further activated by ADP. Ks for ADP is decreased fivefold.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H. Identification of an essential, reactive histidine in pig heart mitochondrial malate dehydrogenase. Eur J Biochem. 1970 Sep;15(3):562–567. doi: 10.1111/j.1432-1033.1970.tb01041.x. [DOI] [PubMed] [Google Scholar]
- Apitz-Castro R., Suarez Z. Structural studies on the active center of alpha-glycerolphosphate dehydrogenase. Biochim Biophys Acta. 1970 Feb 11;198(2):176–182. doi: 10.1016/0005-2744(70)90049-5. [DOI] [PubMed] [Google Scholar]
- Berghäuser J., Falderbaum I., Woenckhaus C. Zuordnung eines essentiellen Histidinrestes der Lactat-Dehydrogenase zur Substratbindungsstelle. Hoppe Seylers Z Physiol Chem. 1971 Jan;352(1):52–58. doi: 10.1515/bchm2.1971.352.1.52. [DOI] [PubMed] [Google Scholar]
- Bond J. S., Francis S. H., Park J. H. An essential histidine in the catalytic activities of 3-phosphoglyceraldehyde dehydrogenase. J Biol Chem. 1970 Mar 10;245(5):1041–1053. [PubMed] [Google Scholar]
- Di Franco A., Iwatsubo M. Complexes transitoires dans la catalyse de la glutamate déshydrogénase. Biochimie. 1971;53(2):153–159. doi: 10.1016/s0300-9084(71)80047-0. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Ingram V. A. Ionic properties of an essential histidine residue in pig heart lactate dehydrogenase. Biochem J. 1973 Apr;131(4):729–738. doi: 10.1042/bj1310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Roberts P. A., Wallis R. B. The site at which 4-iodoacetamidosalicylate reacts with glutamate dehydrogenases. Biochem J. 1973 May;133(1):165–171. doi: 10.1042/bj1330165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Stinson R. A. Reactivity of the essential thiol group of lactate dehydrogenase and substrate binding. Biochem J. 1970 Nov;120(2):289–297. doi: 10.1042/bj1200289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huc C., Olomucki A., Lê-Thi-Lan, Dang-Ba-Pho, Nguyen-Van-Thoai Essential histidyl residues of octopine dehydrogenase. Eur J Biochem. 1971 Jul 29;21(2):161–169. doi: 10.1111/j.1432-1033.1971.tb01452.x. [DOI] [PubMed] [Google Scholar]
- Kallos J., Shaw K. P. Covalent attachment of diethylstilbestrol to glutamate dehydrogenase: implications for allosteric regulation. Proc Natl Acad Sci U S A. 1971 May;68(5):916–919. doi: 10.1073/pnas.68.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior W. B., Jr, Fahrney D. Ethoxyformylation of proteins. Reaction of ethoxyformic anhydride with alpha-chymotrypsin, pepsin, and pancreatic ribonuclease at pH 4. Biochemistry. 1970 Jan 20;9(2):251–258. doi: 10.1021/bi00804a010. [DOI] [PubMed] [Google Scholar]
- Rippa M., Pontremoli S. Evidence of a critical histidine residue in 6-phosphogluconate dehydrogenase from Candida utilis. Biochemistry. 1968 Apr;7(4):1514–1518. doi: 10.1021/bi00844a038. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Yielding K. L., Curran J. F., Summers M. R., Bitensky M. W. The dependence of the substrate specificity on the conformation of crystalline glutamate dehydrogenase. J Biol Chem. 1965 Oct;240(10):3793–3798. [PubMed] [Google Scholar]
- Wallis R. B., Holbrook J. J. The effect of modifying lysine-126 on the physical, catalytic and regulatory properties of bovine liver glutamate dehydrogenase. Biochem J. 1973 May;133(1):173–182. doi: 10.1042/bj1330173. [DOI] [PMC free article] [PubMed] [Google Scholar]


