Abstract
Background
Colonic stenosis in Crohn’s disease (CD) is uncommon, and data on surgery-free survival are limited. This study aimed to determine surgery-free survival rates and identify associated factors.
Patients and methods
A retrospective study was conducted from 2003 to 2022, including patients with CD complicated by colonic stenosis. Patients with uncertain diagnoses or follow-up periods of less than six months were excluded.
Results
Fifty-six patients were included (median age 44 years [range 14-65], male-to-female ratio = 0.93). Surgery-free survival rates were 58.9% at 6 months, 43.7% at 2 years, and 31.7% at 5 years, with an average surgery-free survival of 46.7 months. Univariate analysis showed that joint manifestations (p = 0.01), corticosteroids (p = 0.02), anti-TNF alpha (p = 0.02), salicylates (p = 0.02), and azathioprine (p = 0.01) increased surgery-free survival. Complications such as collections or internal fistulas (p = 0.03), parietal ulceration on imaging (p = 0.01), and acute intestinal obstruction (p = 0.01) were associated with reduced surgery-free survival. In multivariate analysis, biologic therapy was the only independent protective factor against surgery (p = 0.001, OR = 0.19).
Conclusion
The early introduction of biologic therapy is crucial for increasing surgery-free survival in patients with colonic stenosis in CD, given the limited effectiveness of conventional treatments.
Keywords: Inflammatory bowel disease, Crohn’s disease, colonic stricture, surgery, survival, anti TNF alpha therapy, medical treatment, prognostic
ARTICLE HIGHLIGHTS
Colonic stenosis in CD is rare and lacks standardized management.
Data on survival without surgery and associated factors are scarce.
Study aims to determine surgery-free survival rates and identify influencing factors.
Retrospective study on ileocolonic or colonic CD with colonic stenosis.
Inclusion period: January 2003 to December 2022.
Exclusions: Uncertain CD, tuberculosis, cancer, dysplasia
56 patients included; median age at diagnosis = 44 years; male-to-female ratio = 0.93.
Surgery-free survival: 58.9% at 6 months, 43.7% at 2 years, 31.7% at 5 years; average = 46.7 months (3.9 years).
Univariate analysis linked joint manifestations, corticosteroids, IFX, ADA, salicylates, AZA to increased surgery-free survival. Complications, parietal ulceration, acute intestinal obstruction associated with reduced surgery-free survival.
Multivariate analysis identified anti TNF alpha as the only independent protective factor against surgery (p = 0.001, OR = 0.19).
Introduction
Crohn’s disease (CD) is a chronic inflammatory condition of the digestive tract characterized by segmental and transmural inflammation [1]. Typically, patients are diagnosed with an inflammatory phenotype, with up to two-thirds progressing to a stenosing and/or perforating form over the course of the disease [2–5]. One-third of patients with CD develop intestinal stenosis during disease progression [6]. This can occur in any segment of the gastrointestinal tract, with preferential localization of the terminal ileum and ileocolonic anastomoses [7,8]. Colonic stenosis can be inflammatory or fibrous; however, this dichotomy is controversial because both entities often overlap [9]. In this context, the clinical, biological, and radiological characteristics of stenosis can help practitioners identify the inflammatory nature of the stenosis and differentiate it from fibrous stenosis. The management of colonic stenosis poses a challenge due to its complexity and the lack of standardized approaches resulting from the limited number of studies addressing this complication. Present therapeutic strategies often draw from similarities with ileal stenoses; however, a notable difference lies in the central role of the risk of neoplasia in managing colonic stenoses [10]. Moreover, data on surgery-free survival, especially without resorting to surgical intervention, are limited, as are the associated factors. Shedding light on these data and identifying these factors could facilitate patient management and enable a more precise selection of patients eligible for medical treatment. In this regard, the aims of our study were as follows:
Determine surgery-free survival rates in patients with CD complicated by colonic stenosis.
Identify factors influencing surgery-free survival in these patients.
Materials and methods
We conducted a retrospective study at the gastroenterology department spanning from January 2003 to December 2022. The study involved the comprehensive review of patient records for individuals undergoing monitoring due to colonic or ileocolonic CD with associated colonic stenosis.
The diagnosis of CD was established through a comprehensive assessment, incorporating clinical, endoscopic, radiological, and histological criteria. The localization of CD was categorized based on the Montreal classification, encompassing purely colonic (L2) or ileocolonic (L3) involvement, with or without proximal extension (L4).
Confirmation of colonic stenosis diagnosis occurred when there was evidence of colonic lumen narrowing, either passable with friction or impassable by an adult colonoscope. Additionally, confirmation was obtained through cross-sectional imaging conducted during an episode of acute intestinal obstruction or intraoperatively.
Clinical Obstructive Signs: Obstructive pain or sub-occlusive syndromes using CDOS
Inclusion criteria
All patients with colonic or ileocolonic CD presenting with colonic stenosis were included.
Non-inclusion criteria
Patients with an uncertain diagnosis of CD, specifically those with diagnostic uncertainty related to tuberculosis or cancer (adenocarcinoma or dysplasia) were not included in the analysis.
Exclusion criteria
Patients with a follow-up period of less than six months from the diagnosis of colonic stenosis were excluded.
Data collection
Demographic, clinical, CD assessment, stenosis characteristics, and treatment data were gathered for every patient.
Statistical analysis
The SPSS software was utilized for statistical analysis, encompassing descriptive analysis. Survival analysis was conducted using Kaplan–Meier method with survival curves. Univariate analysis, examining factors individually through the log-rank test, was employed to investigate factors influencing surgery-free survival. Additionally, a multivariate Cox proportional hazard regression analysis was carried out to identify independent factors associated with the need for surgery. Statistical significance was considered at a p-value of 0.05.
Ethics statement
This study did not present any conflicts of interest. Strict anonymity and confidentiality of the collected individual data were maintained throughout the study, and we adhered to the recommendations of the ethics committee of La Rabta Hospital.
Results
Patient characteristics
The study included 56 patients with colonic stenosis (Figure 1). The median age of the patients was 44 years [14-65 years]. The sex ratio (men/women) was 0.93. Eight patients were active smokers. Thirteen patients (23%) had at least one comorbidity.
Figure 1.
Flow chart describing the distribution of study patients.
Crohn’s disease assessment
At the time of CD diagnosis, the phenotype was non-fistulizing/non-stricturing phenotype (B1) in 40 cases (71%), stricturing phenotype (B2) in 12 cases (21%), and fistulizing phenotype (B3) in 4 cases (7%). The disease was ileocolonic in 31 cases (55%) and colonic in 25 cases (45%). Upon the diagnosis of colonic stenosis, 29 patients (52%) exhibited active disease, with severity noted in 2 cases. Anoperineal involvement was present in 25 patients (44.6%), and 73% had experienced at least one flare-up before the diagnosis of colonic stenosis.
Among the 56 patients, 4 had a history of prior intestinal resection before colonic stenosis: ileocecal resection with ileocolonic anastomosis in 2 cases and segmental ileal resection with ileo-ileal anastomosis in 2 cases. Regarding treatment, 41 patients (73.2%) were already under treatment before the diagnosis of colonic stenosis, with percentages as follows: 64.3% on azathioprine (AZA) (36 patients), 64.3% on corticosteroids (36 patients), 50% on salicylates (28 patients), and 16.1% on anti-TNF alpha (Infliximab (IFX) or adalimumab (ADA) (9 patients).
Characteristics of colonic stenosis
Colonic stenosis developed during CD in 75% of cases (n = 42), with an average delay of 4.6 years, ranging from 1 month to 19 years. In 14 patients (25%), colonic stenosis was inaugural. The manifestations of colonic stenosis were nonspecific in 34 cases (60%), characterized by a subocclusive syndrome in 14 cases (25%), and acute intestinal occlusion in 4 cases (7%).
Ileocolonoscopy was conducted on 52 patients (92.9%). In all instances, it played a crucial role in diagnosing the stenosis, revealing a non-traversable narrowing of the colonic lumen in 46 cases (88.5%) and a passable narrowing in 6 cases (11.5%). A solitary stenosis was identified in 48 patients (85.7%), while two stenoses were observed in 4 patients (7.1%). Stenosis locations were as follows: sigmoid in 20 cases (35.7%), right colon in 17 cases (39.1%), rectum in 6 cases (10.7%), left colon in 5 cases (8.9%), and transverse in 4 cases (7.1%).
Anatomopathological examination of colonic biopsies revealed chronic inflammatory lesions in 48 cases (82.7%), granulomas in 5 cases (8.9%).
All patients underwent cross-sectional imaging, with a coloscanner used in the majority of cases (n = 42) for enhanced stenosis characterization. MRI was employed in only 14 patients (25%). Colonic stenosis was singular in 38 patients and multiple in 18 patients.
Radiological signs of inflammation were observed in 52 patients. Among the complications associated with stenosis, there were 14 instances, including 6 collections and 8 fistulas. Upstream dilation of the stenosis occurred in only 5 cases, while 51 cases exhibited no pre-stenotic dilation. The average length of the stenosis was 6 cm, ranging from 14 to 180 mm.
Therapeutic modalities for colonic stenosis
Medical treatment for the stenosis was prescribed in 34 cases (60.7%) for patients with active disease, passable stenoses, unique stenoses, and no signs of malignancy in imaging and histology, The treatment regimen included oral corticosteroids in 23 cases (41%) and anti-TNF alpha agents in 18 patients (32.1%) associated to azathioprine. Among them, 14 patients received IFX at an induction dose of 5 mg/kg, followed by maintenance every 8 weeks for those who responded well. Additionally, 6 patients were administered ADA, with two receiving it after the initial failure of IFX. The ADA dosage was 160 mg at week 0, 80 mg at week 2, and then 40 mg for maintenance every two weeks in case of a positive response.
The treatment was successful in 14 cases and failed in 20 cases which were then referred to surgery
Endoscopic dilation was performed in 3 cases (5.4%), proving successful in two instances. One patient underwent a single dilation session, coupled with IFX optimization, while the other patient underwent three dilation sessions.
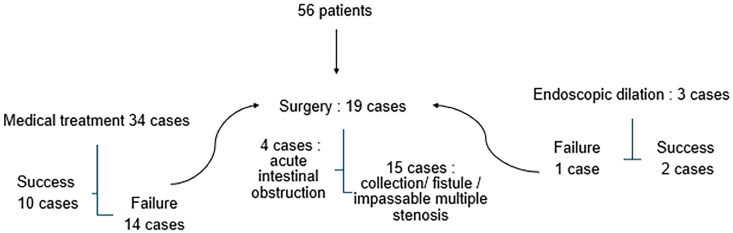
Surgery was chosen at the diagnosis of stenosis for 19 cases (33.9%): 4 cases due to acute intestinal obstruction and 15 cases due to collections, fistulas, or multiple impassable stenoses (Figure 2).
Figure 2.
Chart explaining treatment option.
Throughout the follow-up period, surgery became necessary for 71.4% of patients, with 33.9% undergoing surgery at the time of diagnosis and an additional 37.5% after attempting medical treatment or endoscopic dilation. The surgery rates varied, with 60% following corticosteroid treatment, 33% after anti TNF alpha, 62.5% after AZA, and 50% after salicylates. On average, the time elapsed from the initiation of corticosteroid therapy to surgery was 21 months, with a median of 12 months (ranging from 3 months to 17.7 years).
Surgical specimen results
Of the 48 cases that underwent biopsies before surgery, no signs of malignancy were detected. However, one case (2%) showed high-grade dysplasia in the surgical specimen.
Associated factors for surgery-free survival
Univariate analysis
The factors significantly associated with an increase in surgery-free survival duration were:
The presence of articular manifestations (p = 0.01)
Use of corticosteroids (p = 0.02)
Use of anti TNF alpha (p = 0.02) ((IFX (p = 0.03), ADA (p = 0.03))
Use of salicylates (p = 0.02)
The use of AZA (p = 0.01)
The factors significantly associated with a reduction in surgery-free survival duration were:
The presence of complications (collections and internal fistulas) (p = 0.03)
The presence of parietal ulceration on imaging (p = 0.01)
Acute intestinal obstruction (p = 0.01)
The results of the univariate study are summarized in (Table 1).
Table 1.
Univariate analysis.
| Parameter | Average survival in months | P |
|---|---|---|
| Sex | ||
| Male (n = 27) | 50.8 | 0.82 |
| Female (n = 29) | 35.9 | |
| Tobacco | ||
| Yes (n = 8) | 47.5 | 0.57 |
| No (n = 48) | 32.9 | |
| Comorbidity | ||
| Yes (n = 13) | 58 | 0.95 |
| No (n = 43) | 42 | |
| Joint manifestations | ||
| Yes (n = 6) | 114.4 | 0.01 |
| No (n = 50) | 33.3 | |
| Cutaneous manifestations | ||
| Yes (n = 3) | 40.3 | 0.72 |
| No (n = 53) | 30 | |
| Location of the disease | ||
| Colonic (n = 25) | 52.5 | 0.41 |
| Ileo-colonic (n = 31) | 38.7 | |
| Behaviour | ||
| Stricturing (n = 39) | 45.2 | 0.92 |
| Stricturing et penetrating (n = 17) | 42.5 | |
| Anoperineal manifestations | ||
| Yes (n = 25) | 54.6 | 0.16 |
| No (n = 31) | 32.5 | |
| Previous flare | ||
| Yes (n = 41) | 41.2 | 0.44 |
| No (n = 15) | 52.1 | |
| Number of stenoses | ||
| Single (n = 48) | 45.9 | 0.40 |
| Multiples (n = 4) | 65 | |
| Nonspecific signs | ||
| Yes (n = 34) | 45.9 | 0.65 |
| No (n = 22) | 39.5 | |
| Sub-obstructive syndrome | ||
| Yes (n = 14) | 62.3 | 0.72 |
| No (n = 42) | 52.9 | |
| Acute intestinal obstruction | ||
| Yes (n = 4) | 0 | 0.01 |
| No (n = 52) | 105 | |
| Granuloma | 61 | 0.07 |
| Yes (n = 5) | 26 | |
| No (n = 45) |
Multivariate analysis
The only independent predictive factor against recourse to surgery was biotherapy treatment with an OR = 0.19[0.03-0.94].
Survival of the patients
The surgery-free survival rate was 58.9% at 6 months, 43.7% at 2 years, 31.7% at 5 years, and only 18% at 10 and 15 years (Figures 3 and 4). The average surgery-free survival was 46.7 months, equivalent to 3.9 years.
Figure 3.
(a) Survival curves. (b) Survival curves.
Figure 4.
Survival curve without surgery.
Discussion
In this study, 71.4% of patients required surgery: 33.9% at the time of diagnosis and 37.5% after attempting medical treatment or endoscopic dilation. The surgery rates were 60% after corticosteroid treatment, 33% after anti TNF alpha, 62.5% after AZA, and 50% after salicylates. Surgery-free survival rates were 58.9% at 6 months, 43.7% at 2 years, and 31.7% at 5 years, with an average surgery-free survival of 46.7 months, equivalent to 3.8 years. Independent factors significantly associated with an increase in surgery-free survival included the presence of articular manifestations and medical treatment with corticosteroids, IFX, ADA, salicylates, and AZA.
Indeed, the management of colonic stenosis presents a complex challenge as it lacks standardization due to its heterogeneity and the limited number of dedicated studies. Therapeutic approaches currently in use predominantly rely on a low level of evidence. There has been a scarcity of studies specifically addressing the role of medical treatment in colonic strictures. Strictures were often excluded from most clinical trials, and concerns about the risk of cancer further limited available data on the effectiveness of medical treatment [11,12].
Regarding risk cancer, it is eight times higher in the presence of colonic stenosis in CD. Between 1992 and 2014, GETAID recorded 12.013 patients with IBD who underwent surgery. Among these, 248 cases involved colonic stenosis due to CD. Preoperative biopsies of these stenoses were negative, but a 2.4% incidence of malignancy (dysplasia or cancer) was noted in the surgical specimens [13,14]. Risk factors for malignancy associated with colonic stenosis in Crohn’s disease include being over 50 years old, having inactive disease, and having a short stenosis [15]. In the study, we recorded one case of high-grade dysplasia in surgical specimens, despite negative preoperative biopsies
The medical approach for colonic stricture encompassed corticosteroid therapy, AZA, and anti-TNF agents. In this study, clinical remission was attained in 40% of patients treated with corticosteroids, leading to surgery in 60% of cases. It’s worth noting that the efficacy of corticosteroid therapy in colonic strictures during CD has not been specifically evaluated in dedicated studies but rather in general studies focusing on the short-term effectiveness of these agents in inducing remission in CD. A retrospective cohort study by Samimi et al. [16] examined the effectiveness of medical treatment for CD, initiating corticosteroid therapy in 31% of patients and assessing its impact on days 30, 90, and 180. In cases where CD presented as stricturing, corticosteroid therapy was found effective in 41% of patients by day 180, with 50% eventually undergoing surgery. These findings align with the outcomes observed in our study. Additionally, a study investigating the efficacy of IFX for treating digestive strictures in CD revealed that the sole independent factor associated with a complete response to this treatment was the introduction of prednisone or an escalation in its doses during anti-TNF alpha therapy [17].
Regarding AZA, in our series, 72% of patients were treated with AZA. We observed that immunosuppressants significantly improved surgery-free survival, which was 91.3% at 6 months and 64.6% at 2 years for patients treated with AZA compared with 33.9% at 6 months and 26% at 2 years for untreated patients (p = 0.01). Our findings are in line with a study published in 2019. A Japanese team followed 53 patients with symptomatic stenosis treated medically. The association with AZA was associated with a reduced risk of surgical resection, with a p-value of 0.03 in univariate analysis [18]. The use of AZA is not statistically significant after 5 years of follow-up and did not emerge as a protective factor in the multivariate analysis.
In this study, anti TNF alpha treatment was the only independent predictive factor against surgery. The long follow-up period explains the variability in treatment choices over time. Surgery was considered the safest option, and anti-TNF agents were only used when there were poor prognostic factors, such as rheumatologic manifestations [19,20].
By reviewing the literature, the effectiveness of biological treatments on colonic strictures has never been studied. The current data are from studies that included patients with ileal strictures. The prospective CREOLE cohort from GETAID showed a response to ADA in 2/3 of patients treated for symptomatic ileal stenosis [21]. This strategy is likely to be more effective if the inflammatory component is predominant, as evidenced by the factors associated with its effectiveness. Another series involving 241 patients, including "low-risk strictures" documented by enterography-MRI, showed that the use of biological therapy halved the need for surgery during an average follow-up of 607 days, suggesting that early use of these agents could modify the natural course of the disease [22]. In line with our results, Campos et al. [23] revealed in a retrospective study including 84 patients with documented CD-associated stricture that combination therapy (anti-TNF alpha and AZA) was a protective factor against medical treatment failure (60 months). Similarly, Bouhnik et al. [21] demonstrated that combination therapy (ADA and AZA) was more effective than ADA alone in treating inflammatory-like strictures.
The lack of data on this subject could be explained by the theory that anti-TNF alpha agents might increase the risk of stenosis due to accelerated healing processes, leading to marked architectural changes in the intestinal wall with potential scarring and fibrosis [24]. Most randomized controlled trials investigating the efficacy of anti-TNF alpha agents in CD have thus excluded patients with digestive strictures based on prior observational studies suggesting the occurrence of new strictures or worsening of preexisting ones under anti-TNF alpha treatment. Indeed, a study involving 76 patients with refractory CD demonstrated clinical and endoscopic improvement in 67 patients (88%) after IFX infusion [25]. However, 7 patients (10%) developed new digestive strictures requiring hospitalization and surgical intervention. The authors observed that the onset of IFX action was parallel to the appearance of obstructive symptoms. Moreover, a pilot open-label study [24] by Louis et al. in patients with symptomatic strictures and refractory CD demonstrated the ineffectiveness of IFX. The study was prematurely terminated as the predefined safety thresholds for more than two surgical interventions in the first five patients were reached.
As for salicylates, they are minimally effective in CD, and their use may be debated in mild colonic flares of the disease, with modest efficacy in preventing CD recurrence after surgical intervention [26]. In this study, the surgery-free survival rate was 83.3% at 6 months and 2 years for patients treated with salicylates, compared with 55.1% at 6 months and 37.5% at 2 years for untreated patients (p = 0.02). No studies on this subject were found in the literature. In fact, all patients who received salicylates were on combination therapy or AZA. Therefore, the effect of salicylates on treatment efficacy cannot be independently assessed.
Regarding extraintestinal manifestations, 20% of our patients exhibited such manifestations, with a notable predominance of joint involvement. This rate is consistent with literature reports, though it varies across different studies. with frequencies ranging from 6% to 49% [27]. Indeed, there is a difference in the definition of extra-intestinal involvement from one series to another. The prevalence and incidence of extra-intestinal manifestations, therefore, depend closely on the locations considered in each definition [28].
In our study, the presence of joint involvement significantly influenced survival without surgery, which can be explained using Anti-TNF alpha as maintenance therapy for joint involvement.
It is important to note that only 6 patients had upper digestive tract involvement in our population. This small number weakens the statistical test.
Aside from highlighting objective signs of inflammation, cross-sectional imaging allows for the characterization of stenoses in terms of their location, extent, lumen diameter, upstream dilation, and associated complications such as abscesses and enteric fistulas.
The gold standard for effectively evaluating colonic stricture is CT colonography. Cross-sectional imaging is then essential in order to not only assess the number of strictures and their length but also to screen for complications [such as a fistula] or any signs of malignancy [29–31].
Large bowel MRI can be useful for detecting and characterizing colonic stenoses. It can be performed without colonic distension and with minimal preparation. The sensitivity and specificity of MRI are reported to be between 75% and 91%, and up to 100%, respectively. However, it has limitations such as artifacts caused by fecal matter, which can affect its accuracy. MRI is not included in the ECCO recommendations [32,33].
Intestinal ultrasound is effective for detecting colonic inflammation, with sensitivity and specificity rates of 84% and 92%, respectively. It is also quite useful for identifying colonic stenosis, with sensitivity ranging from 75% to 100% and specificity between 90% and 93%. However, the sensitivity may vary depending on the location of the stenosis, with lower effectiveness for rectal stenoses. The main limitation of intestinal ultrasound is its operator dependence, and it is generally not recommended [34–36].
In our study, predictors of survival without surgery included the absence of enteric fistulas and deep collections visible on cross-sectional imaging. Additionally, the presence of wall ulceration was identified as a significant factor affecting survival without surgery. These findings support the hypothesis that uncontrolled active inflammation is a precursor to a need for surgery.
In most studies evaluating medical treatment for digestive stenoses in Crohn’s disease (CD), the presence of enteric fistulas was an independent predictor of the need for surgery. Campos et al. examined cross-sectional imaging data to predict treatment failure [23].
Out of 55 cross-sectional images taken before treatment initiation, 66% of patients experienced treatment failure, and the presence of fistulas detected by imaging was an independent predictor of this failure. Bouhnik et al. [21] also demonstrated that the success of ADA treatment for inflammatory stenoses within 24 months of treatment initiation was strongly associated with the absence of enteric fistulas at the time of stenosis diagnosis. More recently, Bossuyt et al. [37] reported on a large cohort of over 900 patients with CD and digestive stenosis, whether symptomatic or not. They developed the "The BRACARDI Risk Model," which stratifies the risk of requiring surgery regardless of the type of medical treatment, with the fistulizing phenotype being a determining factor for intestinal resection.
The presence of fistulas associated with the stenosis is independent of the inflammation/fibrosis dichotomy, which might influence the success or failure of medical treatment. Additionally, a strong association between pre-stenotic dilation and the need for surgery was revealed by some study. The length of the stenosis being less than 12 cm, was an independent predictor of the success of medical treatment [21].
In our study, stenosis lengths ranged from 1.4 to 18 cm and did not influence survival without surgery. Additionally, it is noteworthy that colonic stenosis was predominantly singular in most cases. This observation can be attributed to the segmental nature of inflammation in Crohn’s disease, which typically affects a single segment of the colon, resulting in stenosis.
For the pre-stenotic dilation, we found only 5 cases with dilation, whereas 51 cases had no pre-stenotic dilation to assess and exclude this possibility in the patients imaging techniques were employed, including CT scans, MRIs, endoscopic evaluations and biopsy and histological analysis. Patients were closely monitored over time to observe the progression of the stenotic lesions. The stability or regression of these lesions, along with the absence of typical cancer-related symptoms, provided additional evidence against a neoplastic nature.
In a case-control study including 86 patients, Mao et al. [38] aimed to identify risk factors for surgery within 3 years of diagnosing a digestive stenosis via endoscopy. The rate of surgery was 38.4%. Obstructive signs (P = 0.012) and a CDAI score > 220 (P = 0.015) were independent predictors of surgery in multivariate analysis. Similarly, in an Australian study of 136 patients with ileal stenosing CD, hospitalization for acute bowel obstruction was a predictive factor for progression to surgery in multivariate analysis (OR = 2.05; 95% CI [0.96-4.36]) [39].
Our results align with these findings. Indeed, all patients who experienced acute bowel obstruction underwent surgery. The presence of acute bowel obstruction significantly influenced survival without surgery (with a 6-month survival rate without surgery being zero). These results can be explained by the fact that patients with acute bowel obstruction are often promptly directed toward surgical resection if rapid resolution with corticosteroids is not achieved, due to the severity of their condition.
In CD, ECCO has recommended segmental colectomy after endoscopic dilation failure. Oncologic-type surgery should be suggested for colonic strictures complicating extensive, long-duration colitis. Due to the fear of an underlying complication, stricturoplasty is not recommended for colonic strictures [12,40].
When dysplasia or cancer is evidenced on an endoscopic biopsy, colectomy is obligatory; according to the ECCO guidelines, total proctocolectomy is preferred because of the risk of multifocal lesions or metachronous cancer. For stricture without dysplasia or cancer on biopsies, benefits of colonic resection should be discussed in a multidisciplinary team meeting. Segmental colectomy is preferable when lesions are segmental, affecting less than one-third of the colon [41–43].
Conclusion
In summary, the timely initiation of biological therapy emerges as a critical factor in enhancing surgery-free survival, considering the constrained efficacy of traditional medical approaches for colonic stenosis in CD. Conducting more extensive studies is imperative to pinpoint factors that could streamline patient management, enabling a more precise selection of candidates for medical intervention and consequently extending the duration of surgery-free survival.
Strengths and limitations of the study
This study has some limitations, primarily due to its retrospective nature, which restricted information collection to medical records, resulting in a lack of certain data. The quality of imaging reports also limited the evaluation of several radiological characteristics. Additionally, the retrospective nature exposes the study to various selection biases, while the monocentric nature limits the number of patients, thereby impacting the power of the results.
However, it possesses several strengths. It benefits from significant scope, including a homogeneous population with extensive follow-up, as well as strict inclusion and exclusion criteria that minimized follow-up losses. The results of statistical tests were reliable, allowing for robust and consistent conclusions. The fact that our research was conducted within a single center offers advantages, including consistency in the diagnosis of colonic strictures by a unified medical team and standardized therapeutic practices. To our knowledge, the study stands out by focusing on surgery-free survival and factors associated with colonic strictures in CD, a topic that has been underexplored until now.
Funding Statement
There was no funding.
Authors’ contributions
HA and SL were responsible for the diagnosis and clinical management of the patient. SL, SS and HJ participated in the analysis, supervision, writing of the original draft, reviewing and editing of the manuscript for intellectual content. All authors read and approved the final manuscript.
Ethics statement
The study was performed in accordance with the principles of the Declaration of Helsinki and Its appendices and with local and national laws.
Disclosure statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Aniwan S, Park SH, Loftus EV.. Epidemiology, natural history, and risk stratification of Crohn’s disease. Gastroenterol Clin North Am. 2017;46(3):463–480. doi: 10.1016/j.gtc.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 2.Rieder F, Fiocchi C, Rogler G.. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017;152(2):340–350.e6. doi: 10.1053/j.gastro.2016.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]; * (Provides a comprehensive overview of fibrosis mechanisms in inflammatory bowel diseases, essential for understanding the development of stenosis in Crohn’s disease.)
- 3.Feuerstein JD, Cheifetz AS.. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc. 2017;92(7):1088–1103. doi: 10.1016/j.mayocp.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 4.Bettenworth D, Bokemeyer A, Baker M, et al. Assessment of Crohn’s disease associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review. Gut. 2019;68(6):1115–1126. doi: 10.1136/gutjnl-2018-318081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vrabie R, Irwin GL, Friedel D.. Endoscopic management of inflammatory bowel disease strictures. World J Gastrointest Endosc. 2012;4(11):500–505. doi: 10.4253/wjge.v4.i11.500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62(7):1072–1084. doi: 10.1136/gutjnl-2012-304353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King D, Reulen RC, Thomas T, et al. Changing patterns in the epidemiology and outcomes of inflammatory bowel disease in the United Kingdom: 2000-2018. Aliment Pharmacol Ther. 2020;51(10):922–934. doi: 10.1111/apt.15701 [DOI] [PubMed] [Google Scholar]; * (Presents valuable epidemiological data, supporting insights into trends and outcomes in Crohn’s disease.)
- 8.Van Assche G, Geboes K, Rutgeerts P.. Medical therapy for Crohn’s disease strictures. Inflamm Bowel Dis. 2004;10(1):55–60. doi: 10.1097/00054725-200401000-00009 [DOI] [PubMed] [Google Scholar]; * (Explores therapeutic approaches to strictures, bridging the clinical management with the observed pathological findings)
- 9.Brochard C, Siproudhis L, Wallenhorst T, et al. Anorectal stricture in 102 patients with Crohn’s disease: natural history in the era of biologics. Aliment Pharmacol Ther. 2014;40(7):796–803. doi: 10.1111/apt.12894 [DOI] [PubMed] [Google Scholar]
- 10.Magro M, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on diagnosis and management of ulcerative Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11(6):649–670. doi: 10.1093/ecco-jcc/jjx008 [DOI] [PubMed] [Google Scholar]
- 11.Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14(1):4–22. doi: 10.1093/ecco-jcc/jjz180 [DOI] [PubMed] [Google Scholar]
- 12.Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for diagnostic assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13(2):144–164. doi: 10.1093/ecco-jcc/jjy113 [DOI] [PubMed] [Google Scholar]; ** (Authoritative guideline offering critical diagnostic insights, enabling accurate identification and monitoring of complications like stenosis in Crohn’s disease.)
- 13.Fumery M, Pineton De Chambrun G, Stefanescu C, et al. Detection of dysplasia or cancer in 3.5% of patients with inflammatory bowel disease and colonic strictures. Clin Gastroenterol Hepatol. 2015;13(10):1770–1775. doi: 10.1016/j.cgh.2015.04.185 [DOI] [PubMed] [Google Scholar]
- 14.Wijnands AM, De Jong ME, Lutgens MWMD, et al. Prognostic factors for advanced colorectal neoplasia in inflammatory bowel disease: systematic review and meta-analysis. Gastroenterology. 2021;160(5):1584–1598. doi: 10.1053/j.gastro.2020.12.036 [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki Y, Ribeiro MB, Sachar DB, et al. Malignant colorectal strictures in Crohn’s disease. Am J Gastroenterol (Springer Nature). 1991;86(7):882–885. [PubMed] [Google Scholar]
- 16.Samimi R, Flasar MH, Kavic S, et al. Outcome of medical treatment of stricturing and penetrating Crohn’s disease: a retrospective study. Inflamm Bowel Dis. 2010;16(7):1187–1194. doi: 10.1002/ibd.21160 [DOI] [PubMed] [Google Scholar]
- 17.Pelletier AL, Kalisazan B, Wienckiewicz J, et al. Infliximab treatment for symptomatic Crohn’s disease strictures. Aliment Pharmacol Ther. 2009;29(3):279–285. doi: 10.1111/j.1365-2036.2008.03887.x [DOI] [PubMed] [Google Scholar]
- 18.Maehata Y, Nagata Y, Moriyama T, et al. Risk of surgery in patients with stricturing type of Crohn’s disease at the initial diagnosis: a single center experience. Intest Res. 2019;17(3):357–364. doi: 10.5217/ir.2018.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wada H, Murate K, Nakamura M.. The effects of ustekinumab on small intestinal lesions and stenotic lesions. Nagoya University Graduate School of Medicine, School of Medicine; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menegatti S, Bianchi E, Rogge L.. Anti-TNF therapy in spondyloarthritis and related diseases, impact on the immune system and prediction of treatment responses. Front Immunol. 2019;10:382. doi: 10.3389/fimmu.2019.00382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut. 2018;67(1):53–60. doi: 10.1136/gutjnl-2016-312581 [DOI] [PMC free article] [PubMed] [Google Scholar]; ** (An important study that evaluates the therapeutic role of adalimumab in managing strictures, providing practical insights into treatment outcomes for Crohn’s disease patients with stenotic complications.)
- 22.Buisson A, Joubert A, Montoriol PF, et al. Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn’s disease. Aliment Pharmacol Ther. 2013;37(5):537–545. doi: 10.1111/apt.12201 [DOI] [PubMed] [Google Scholar]
- 23.Campos C, Perrey A, Lambert C, et al. Medical therapies for stricturing Crohn’s disease: efficacy and cross-Sectional imaging predictors of therapeutic failure. Dig Dis Sci. 2017;62(6):1628–1636. doi: 10.1007/s10620-017-4572-4 [DOI] [PubMed] [Google Scholar]
- 24.Louis E, Boverie J, Dewit O, et al. Treatment of small bowel subocclusive Crohn’s disease with infliximab: an open pilot study. Acta Gastro-Enterol Belg. 2007;70(1):15–19. [PubMed] [Google Scholar]
- 25.Vasilopoulos S, Kugathasan S, Saeian K, et al. Intestinal strictures complicating initially successful infliximab treatment for luminal Crohn’s disease. Am J Gastroenterology. 2000;95(9):2503–2503. doi: 10.1111/j.1572-0241.2000.02675.x [DOI] [Google Scholar]
- 26.Ford AC, Khan KJ, Talley NJ, et al. 5-aminosalicylates prevent relapse of Crohn’s disease after surgically induced remission: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(3):413–420. doi: 10.1038/ajg.2010.317 [DOI] [PubMed] [Google Scholar]
- 27.Pittet V, Juillerat P, Mottet C, et al. Cohort profile: the Swiss inflammatory bowel disease cohort study (SIBDCS). Int J Epidemiol. 2009;38(4):922–931. and doi: 10.1093/ije/dyn180 [DOI] [PubMed] [Google Scholar]
- 28.Annese V. A review of extraintestinal manifestations and complications of inflammatory bowel disease. Saudi J Med Med Sci. 2019;7(2):66–73. doi: 10.4103/sjmms.sjmms_81_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amiot A, Bouguen G, Bonnaud G, et al. Clinical guidelines for the management of inflammatory bowel disease: Update of a French national consensus. Dig Liver Dis. 2021;53(1):35–43. doi: 10.1016/j.dld.2020.10.018 [DOI] [PubMed] [Google Scholar]; ** (Provides an updated, evidence-based framework for the management of inflammatory bowel disease, with specific emphasis on clinical decision-making in Crohn’s disease and its complications, including stenosis.)
- 30.Reutemann BA, Turkeltaub JA, Al-Hawary M, et al. Endoscopic balloon dilation size and avoidance of surgery in stricturing Crohn’s disease. Inflamm Bowel Dis. 2017;23(10):1803–1809. doi: 10.1097/MIB.0000000000001181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mang T, Graser A, Schima W, et al. CT colonography: techniques, indications, findings. Eur J Radiol. 2007;61(3):388–399. doi: 10.1016/j.ejrad.2006.11.019 [DOI] [PubMed] [Google Scholar]
- 32.de Jonge CS, Smout AJPM, Nederveen AJ, et al. Evaluation of gastrointestinal motility with MRI: advances, challenges and opportunities. Neurogastroenterol Motil. 2018;30(1):e13257. doi: 10.1111/nmo.13257 [DOI] [PubMed] [Google Scholar]
- 33.Cicero G, Ascenti G, Blandino A, et al. Overview of the large bowel assessment using magnetic resonance imaging: different techniques for current and emerging clinical applications. Curr Med Imaging. 2022;18(10):1031–1045. doi: 10.2174/1573405618666220331111237 [DOI] [PubMed] [Google Scholar]
- 34.Alshammari MT, Stevenson R, Abdul-Aema B, et al. Diagnostic accuracy of non-invasive imaging for detection of colonic inflammation in patients with inflammatory bowel disease: a systematic review and meta-analysis. Diagnostics. 2021;11(10):1926. doi: 10.3390/diagnostics11101926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaban N, Hoad CL, Naim I, et al. Imaging in inflammatory bowel disease: current and future perspectives. Frontline Gastroenterol. 2022;13(e1):e28–e34. doi: 10.1136/flgastro-2022-102117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sagami S, Kobayashi T, Miyatani Y, et al. Accuracy of ultrasound for evaluation of colorectal segments in patients with inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19(5):908–921.e6. doi: 10.1016/j.cgh.2020.07.067 [DOI] [PubMed] [Google Scholar]
- 37.Bossuyt P, Debeuckelaere C, Ferrante M, et al. Risk stratification for surgery in stricturing ileal Crohn’s disease: The BACARDI risk model. J Crohns Colitis. 2018;12(1):32–38. doi: 10.1093/ecco-jcc/jjx110 [DOI] [PubMed] [Google Scholar]
- 38.Mao R, Chen BL, He Y, et al. Factors associated with progression to surgery in Crohn’s disease patients with endoscopic stricture. Endoscopy. 2014;46(11):956–962. doi: 10.1055/s-0034-1390791 [DOI] [PubMed] [Google Scholar]
- 39.Schulberg JD, Wright EK, Holt BA, et al. Magnetic resonance enterography for predicting the clinical course of Crohn’s disease strictures. J Gastroenterol Hepatol. 2020;35(6):980–987. doi: 10.1111/jgh.14908 [DOI] [PubMed] [Google Scholar]
- 40.Fumery M, Yzet C, Chatelain D, et al. Colonic strictures in inflammatory bowel disease: epidemiology, complications, and management. J Crohns Colitis. 2021;15(10):1766–1773. doi: 10.1093/ecco-jcc/jjab068 [DOI] [PubMed] [Google Scholar]
- 41.Bemelman WA, Warusavitarne J, Sampietro GM, et al. ECCO-ESCP consensus on surgery for Crohn’s disease. J Crohns Colitis. 2018;12(1):1–16. doi: 10.1093/ecco-jcc/jjx061 [DOI] [PubMed] [Google Scholar]
- 42.Angriman I, Pirozzolo G, Bardini R, et al. A systematic review of segmental vs subtotal colectomy and subtotal colectomy vs total proctocolectomy for colonic Crohn’s disease. Colorectal Dis. 2017;19:e279–87. [DOI] [PubMed] [Google Scholar]
- 43.Chang CW, Wong JM, Tung CC, et al. Intestinal stricture in Crohn’s disease. Intest Res. 2015;13(1):19–26. doi: 10.5217/ir.2015.13.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.