Abstract
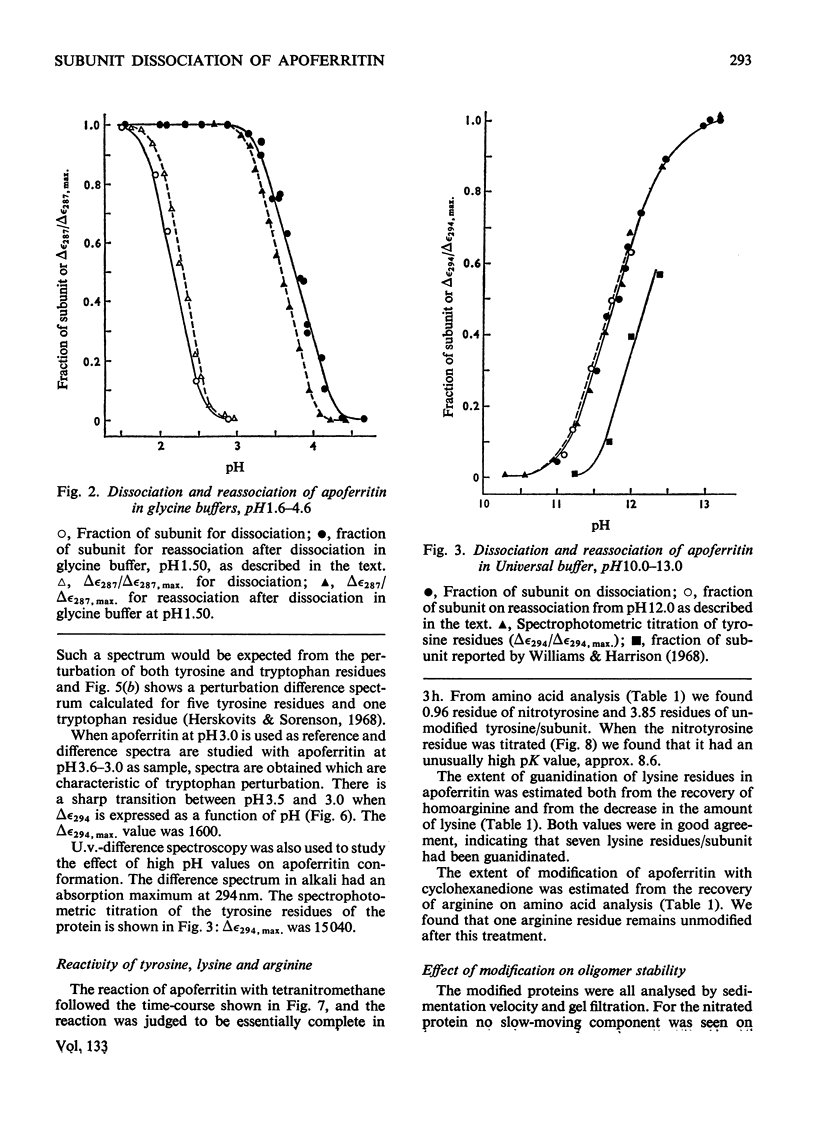
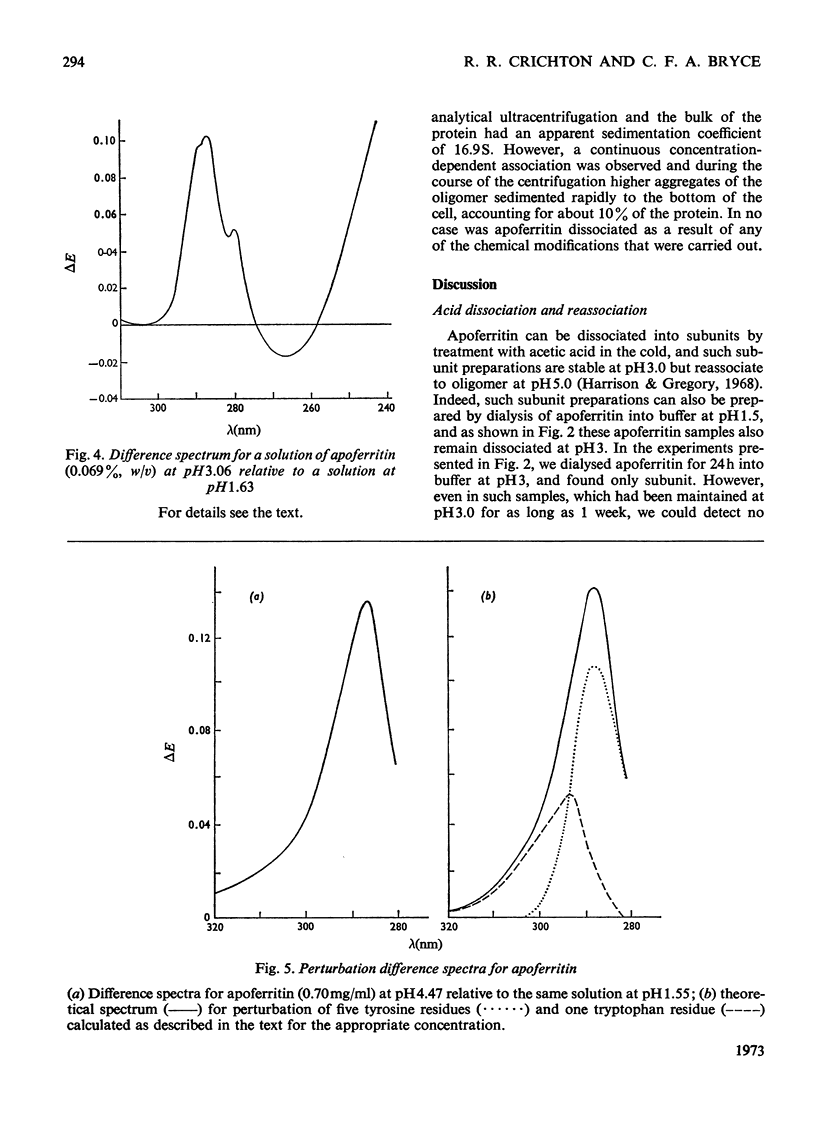
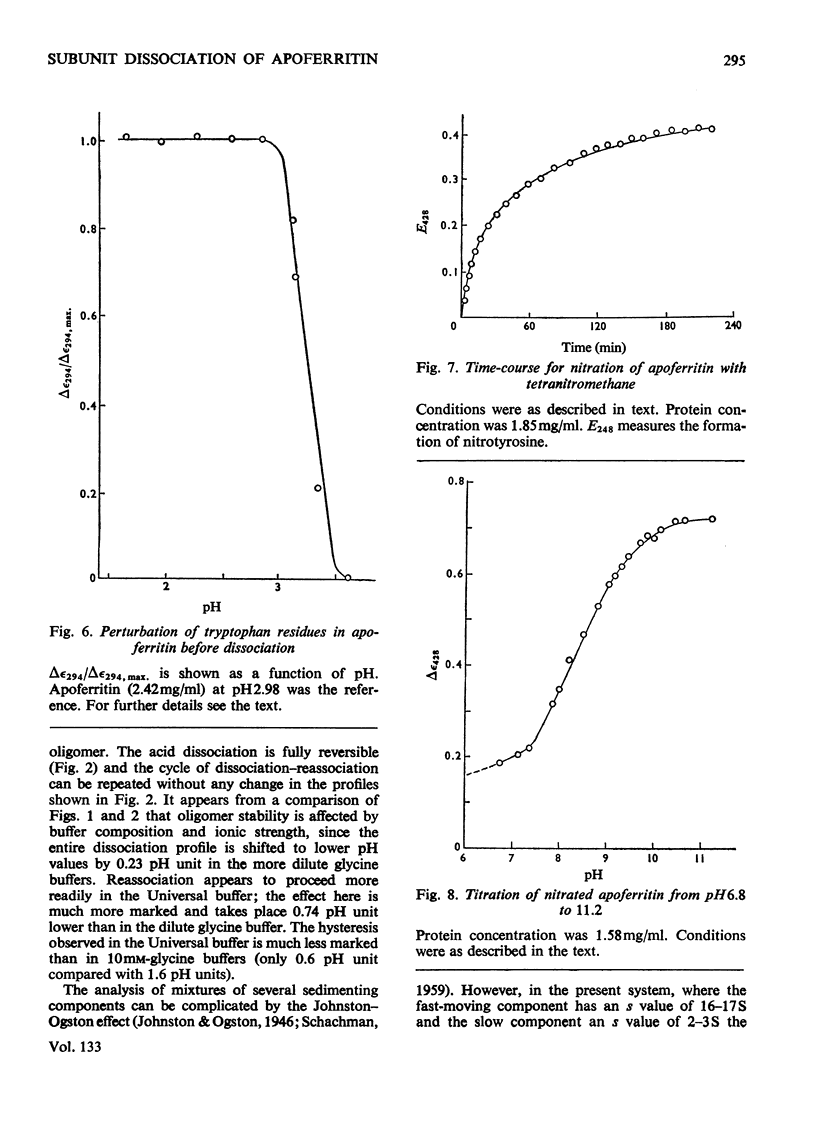
1. The dissociation of horse spleen apoferritin as a function of pH was analysed by sedimentation-velocity techniques. The oligomer is stable in the range pH2.8–10.6. Between pH2.8 and 1.6 and 10.6 and 13.0 both oligomer and subunits can be detected. At pH values between 1.6 and 1.0 the subunit is the only species observed, although below pH1.0 aggregation of the subunits to a particle sedimenting much faster than the oligomer occurs. 2. When apoferritin is first dissociated into subunits at low pH values and then dialysed into buffers of pH1.5–5.0, the subunit reassociates to oligomer in the pH range 3.1–4.3. 3. U.v.-difference spectroscopy was used to study conformational changes occurring during the dissociation process. The difference spectrum in acid can be accounted for by the transfer of four to five tyrosine residues/subunit from the interior of the protein into the solvent. This process is reversed on reassociation, but shows the same hysteresis as found by sedimentation techniques. The difference spectrum in alkali is more complex, but is consistent with the deprotonation of tyrosine residues, which appear to have rather high pK values. 4. In addition to the involvement of tyrosine residues in the conformational change at low pH values, spectral evidence is presented that one tryptophan residue/subunit also changes its environment before dissociation and subsequent to reassociation. 5. Analysis of the dissociation and reassociation of apoferritin at low pH values suggests that this is a co-operative process involving protonation and deprotonation of at least two carboxyl functions of rather low intrinsic pK. The dissociation at alkaline pH values does not appear to be co-operative. 6. Of the five tyrosine residues/subunit only one can be nitrated with tetranitromethane. Guanidination of lysine residues results in the modification of seven out of a total of nine residues/subunit. Nine out of the ten arginine residues/subunit react with cyclohexanedione.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjork I., Fish W. W. Native and subunit molecular weights of apoferritin. Biochemistry. 1971 Jul 20;10(15):2844–2848. doi: 10.1021/bi00791a007. [DOI] [PubMed] [Google Scholar]
- Bryce C. F., Crichton R. R. The catalytic activity of horse spleen apoferritin. Preliminary kinetic studies and the effect of chemical modification. Biochem J. 1973 Jun;133(2):301–309. doi: 10.1042/bj1330301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce C. F., Crichton R. R. The subunit structure of horse spleen apoferritin. I. The molecular weight of the subunit. J Biol Chem. 1971 Jul 10;246(13):4198–4205. [PubMed] [Google Scholar]
- Crichton R. R., Bryce C. F.A. Molecular weight estimation of apoferritin sub-units. FEBS Lett. 1970 Jan 26;6(2):121–124. doi: 10.1016/0014-5793(70)80017-5. [DOI] [PubMed] [Google Scholar]
- Crichton R. R., Eason R., Barclay A., Bryce C. F. The subunit structure of horse spleen apoferritin; the molecular weight of the oligomer and its stability to dissociation by dilution. Biochem J. 1973 Apr;131(4):855–857. doi: 10.1042/bj1310855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton R. R. Ferritin: structure, synthesis and function. N Engl J Med. 1971 Jun 24;284(25):1413–1422. doi: 10.1056/NEJM197106242842506. [DOI] [PubMed] [Google Scholar]
- Crichton R. R., Millar J. A., Cumming R. L., Bryce C. F. The organ-specificity of ferritin in human and horse liver and spleen. Biochem J. 1973 Jan;131(1):51–59. doi: 10.1042/bj1310051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton R. R. Studies on the structure of ferritin and apoferritin from horse spleen. II. Chymotrypsin, subtilisin, cathepsin D and pepsin digestion of ferritin and apoferritin. Biochim Biophys Acta. 1971 Jan 19;229(1):75–82. doi: 10.1016/0005-2795(71)90320-5. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Fuchs S., Anfinsen C. B. The tyrosyl residues at the active site of staphylococcal nuclease. Modifications by tetranitromethane. J Biol Chem. 1968 Sep 25;243(18):4787–4798. [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H. Degradation of tobacco mosaic virus with acetic acid. Virology. 1957 Aug;4(1):1–4. doi: 10.1016/0042-6822(57)90038-7. [DOI] [PubMed] [Google Scholar]
- HOFMANN T., HARRISON P. M. The structure of apoferritin: degradation into and molecular weight of subunits. J Mol Biol. 1963 Apr;6:256–267. doi: 10.1016/s0022-2836(63)80087-x. [DOI] [PubMed] [Google Scholar]
- Harrison P. M., Gregory D. W. Reassembly of apoferritin molecules from subunits. Nature. 1968 Nov 9;220(5167):578–580. doi: 10.1038/220578a0. [DOI] [PubMed] [Google Scholar]
- Herskovits T. T., Sorensen M. Studies of the location of tyrosyl and tryptophyl residues in proteins. I. Solvent perturbation data of model compounds. Biochemistry. 1968 Jul;7(7):2523–2532. doi: 10.1021/bi00847a012. [DOI] [PubMed] [Google Scholar]
- Jaenicke R., Bartmann P. Dissociation-association properties of apoferritin in the milligram and microgram range. Biochem Biophys Res Commun. 1972 Nov 15;49(4):884–890. doi: 10.1016/0006-291x(72)90295-1. [DOI] [PubMed] [Google Scholar]
- Listowsky I., Blauer G., Enlard S., Betheil J. J. Denaturation of horse spleen ferritin in aqueous guanidinium chloride solutions. Biochemistry. 1972 May 23;11(11):2176–2182. doi: 10.1021/bi00761a026. [DOI] [PubMed] [Google Scholar]
- Myers B., 2nd, Glazer A. N. Spectroscopic studies of the exposure of tyrosine residues in proteins with special reference to the subtilisins. J Biol Chem. 1971 Jan 25;246(2):412–419. [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. Intrinsic dissociation constants of aspartyl and glutamyl carboxyl groups. J Biol Chem. 1967 Oct 25;242(20):4731–4735. [PubMed] [Google Scholar]
- Sokolovsky M., Riordan J. F., Vallee B. L. Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966 Nov;5(11):3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- Toi K., Bynum E., Norris E., Itano H. A. Studies on the chemical modification of arginine. I. The reaction of 1,2-cyclohexanedione with arginine and arginyl residues of proteins. J Biol Chem. 1967 Mar 10;242(5):1036–1043. [PubMed] [Google Scholar]
- Tu A. T., Hong B., Solie T. N. Characterization and chemical modifications of toxins isolated from the venoms of the sea snake, Laticauda semifasciata, from Philippines. Biochemistry. 1971 Apr 13;10(8):1295–1304. doi: 10.1021/bi00784a004. [DOI] [PubMed] [Google Scholar]
- Williams M. A., Harrison P. M. Electron-microscopic and chemical studies of oligomers in horse ferritin. Biochem J. 1968 Nov;110(2):265–280. doi: 10.1042/bj1100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder A. F., Gent W. L. Correction of light-scattering errors in spectrophotometric protein determinations. Biopolymers. 1971;10(7):1243–1251. doi: 10.1002/bip.360100713. [DOI] [PubMed] [Google Scholar]
- Wood G. C., Crichton R. R. Optical rotatory dispersion and circular dichroism studies on ferritin and apoferritin. Biochim Biophys Acta. 1971 Jan 19;229(1):83–87. doi: 10.1016/0005-2795(71)90321-7. [DOI] [PubMed] [Google Scholar]


