Abstract
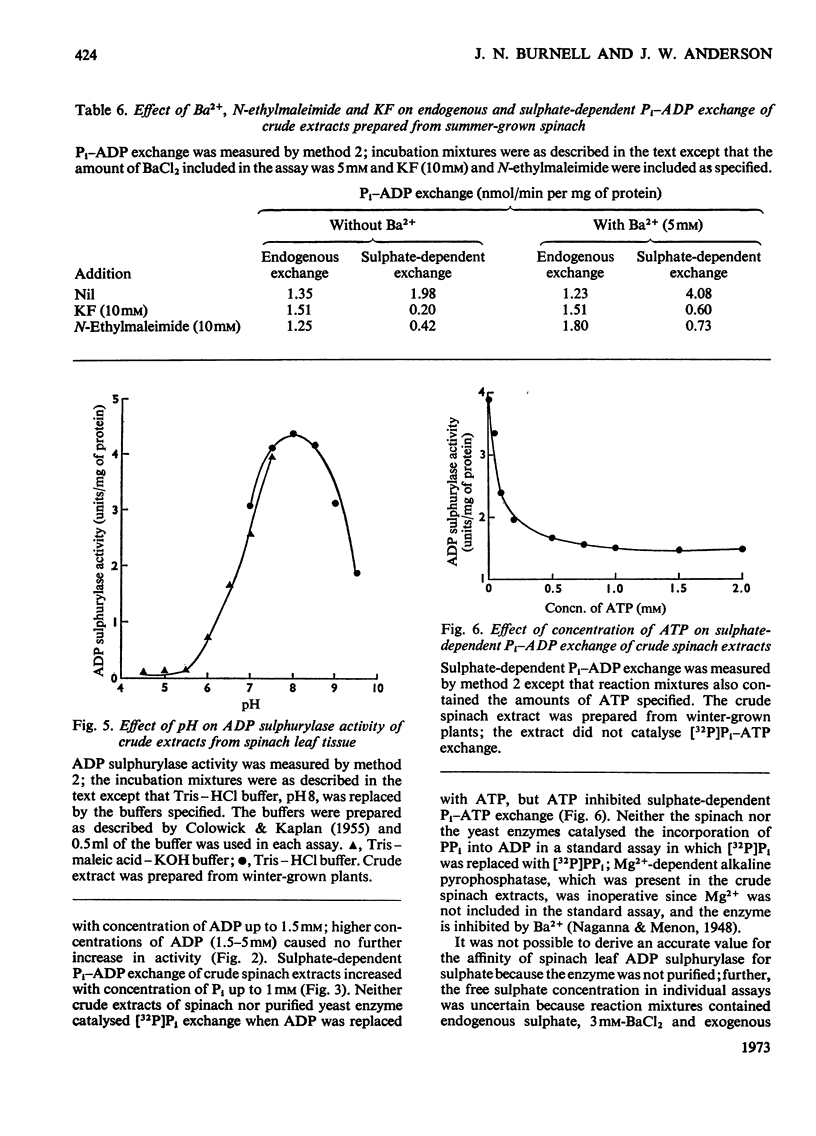
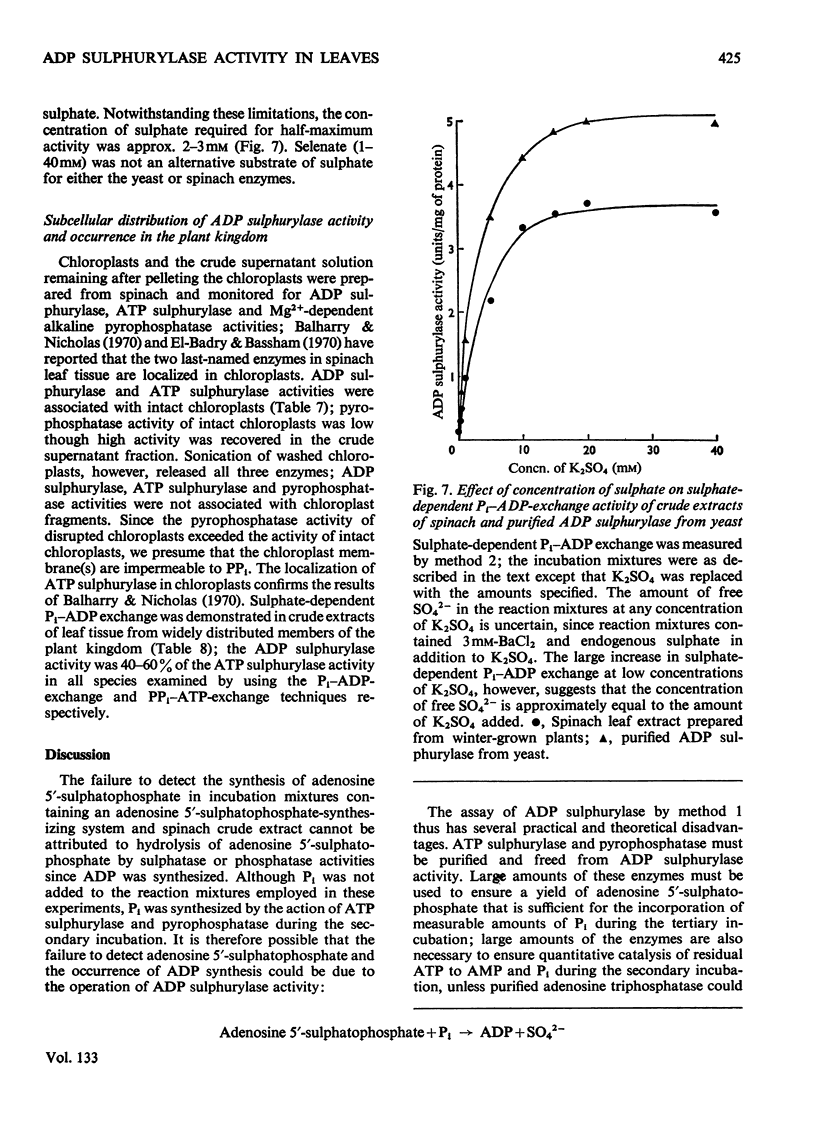
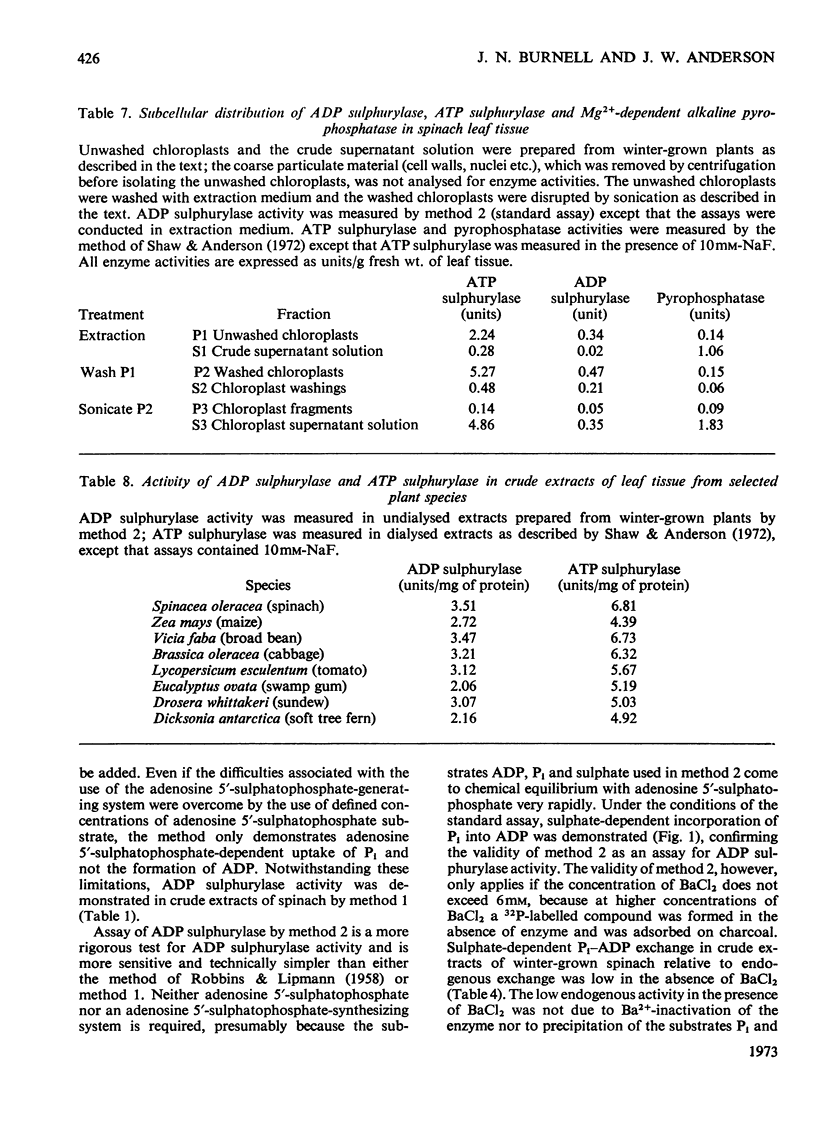
1. A new method is described for the assay of ADP sulphurylase. The method involves sulphate-dependent [32P]Pi–ADP exchange; the method is simpler, more sensitive and more direct than the method involving adenosine 5′-sulphatophosphate-dependent uptake of Pi. 2. ADP sulphurylase activity was demonstrated in crude extracts of leaf tissue from a range of plants. Crude spinach extract catalysed the sulphate-dependent synthesis of [32P]ADP from [32P]Pi; spinach extracts did not catalyse sulphate-dependent AMP–Pi, ADP–PPi or ATP–Pi exchange under standard assay conditions. ADP sulphurylase activity in spinach leaf tissue was associated with chloroplasts and was liberated by sonication. 3. Some elementary kinetics of crude spinach leaf and purified yeast ADP sulphurylases in the standard assay are described; addition of Ba2+ was necessary to minimize endogenous Pi–ADP exchange of the yeast enzyme and crude extracts of winter-grown spinach. 4. Spinach leaf ADP sulphurylase was activated by Ba2+ and Ca2+; Mg2+ was ineffective. The yeast enzyme was also activated by Ba2+. The activity of both enzymes decreased with increasing ionic strength. 5. Purified yeast and spinach leaf ADP sulphurylases were sensitive to thiol-group reagents and fluoride. The pH optimum was 8. ATP inhibited sulphate-dependent Pi–ADP exchange. Neither selenate nor molybdate inhibited sulphate-dependent Pi–ADP exchange and crude spinach extracts did not catalyse selenate-dependent Pi–ADP exchange. 6. The presence of ADP sulphurylase activity jeopardizes the enzymic synthesis of adenosine 5′-sulphatophosphate from ATP and sulphate with purified ATP sulphurylase and pyrophosphatase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. A., Rinne R. W. Influence of age and sulfur metabolism on ATP sulfurylase activity in the soybean and a survey of selected species. Plant Physiol. 1969 Sep;44(9):1241–1246. doi: 10.1104/pp.44.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. W., Rowan K. S. The extraction and assay of aminoacyl-transfer-ribonucleic acid synthetases of tobacco leaf. Biochem J. 1966 Oct;101(1):9–14. doi: 10.1042/bj1010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balharry G. J., Nicholas D. J. ATP-sulphurylase in spinach leaves. Biochim Biophys Acta. 1970 Dec 16;220(3):513–524. doi: 10.1016/0005-2744(70)90282-2. [DOI] [PubMed] [Google Scholar]
- DAVIE E. W., KONINGSBERGER V. V., LIPMANN F. The isolation of a tryptophan-activating enzyme from pancreas. Arch Biochem Biophys. 1956 Nov;65(1):21–38. doi: 10.1016/0003-9861(56)90173-4. [DOI] [PubMed] [Google Scholar]
- Ellis P. An act of love ... or an admission of failure? Euthanasia. Nurs Times. 1992 Sep 9;88(37):34–35. [PubMed] [Google Scholar]
- Grunberg-Manago M., Del Campillo-Campbell A., Dondon L., Michelson A. M. ADP-sulfurylase de levure catalysant un échange entre l'orthophosphate et le phosphate terminal des nucleosides diphosphates. Biochim Biophys Acta. 1966 Jul 20;123(1):1–16. [PubMed] [Google Scholar]
- PECK H. D., Jr The role of adenosine-5'-phosphosulfate in the reduction of sulfate to sulfite by Desulfovibrio desulfuricans. J Biol Chem. 1962 Jan;237:198–203. [PubMed] [Google Scholar]
- Peck H. D. ADENOSINE 5'-PHOSPHOSULFATE AS AN INTERMEDIATE IN THE OXIDATION OF THIOSULFATE BY THIOBACILLUS THIOPARUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1053–1057. doi: 10.1073/pnas.46.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Separation of the two enzymatic phases in active sulfate synthesis. J Biol Chem. 1958 Sep;233(3):681–685. [PubMed] [Google Scholar]
- SPENCER D., WILDMAN S. G. THE INCORPORATION OF AMINO ACIDS INTO PROTEIN BY CELL-FREE EXTRACTS FROM TOBACCO LEAVES. Biochemistry. 1964 Jul;3:954–959. doi: 10.1021/bi00895a019. [DOI] [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Assay of adenosine 5-triphosphate sulfurylase by pyrophosphate exchange. Plant Physiol. 1971 Jan;47(1):114–118. doi: 10.1104/pp.47.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Purification, properties and substrate specificity of adenosine triphosphate sulphurylase from spinach leaf tissue. Biochem J. 1972 Mar;127(1):237–247. doi: 10.1042/bj1270237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]
- el-Badry A. M., Bassham J. A. Chloroplast inorganic pyrophosphatase. Biochim Biophys Acta. 1970 Mar 3;197(2):308–316. doi: 10.1016/0005-2728(70)90042-3. [DOI] [PubMed] [Google Scholar]


