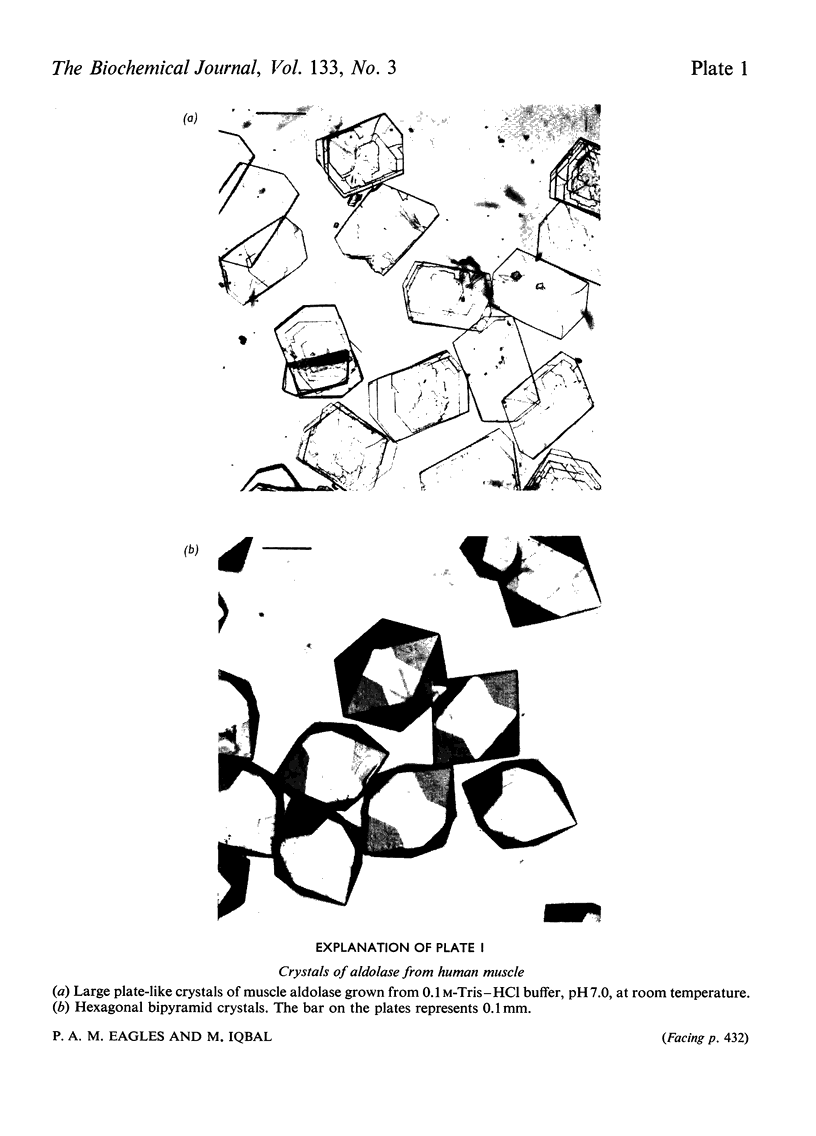
Abstract
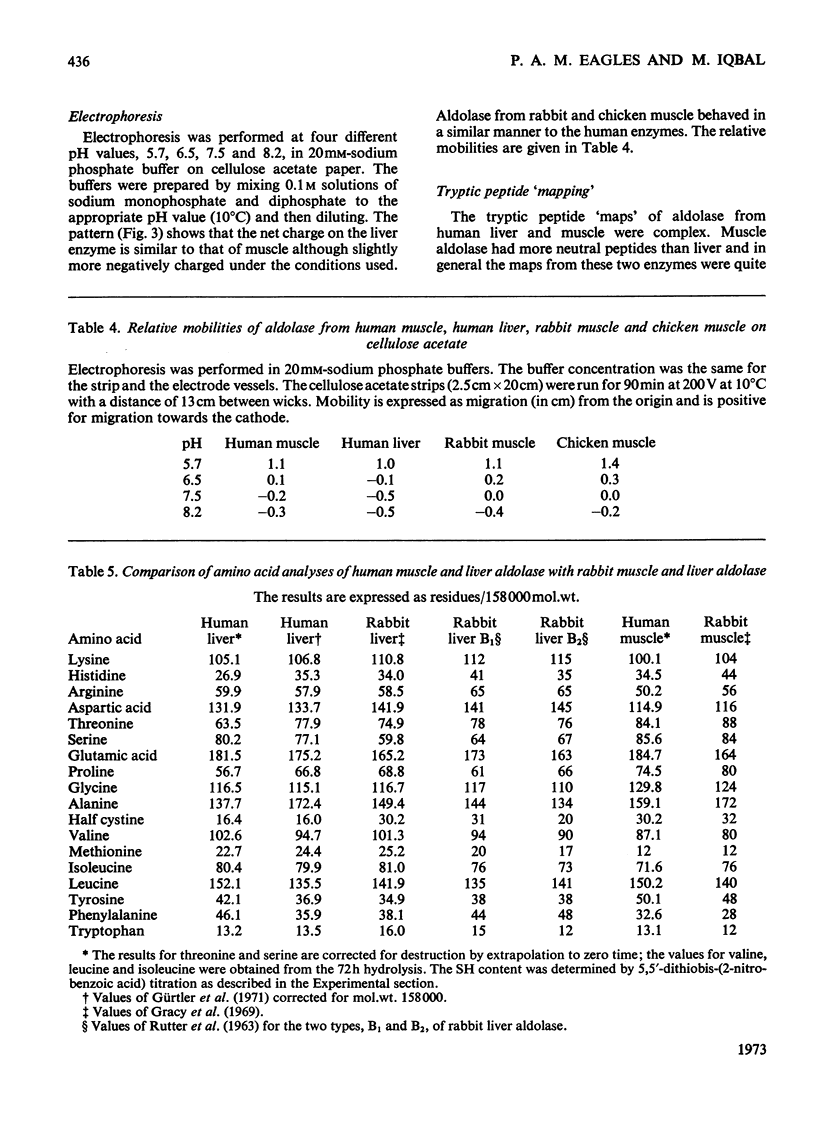
Aldolase was purified from human skeletal muscle and human liver by techniques capable of processing large quantities (10–20kg) of tissue. The methods used also proved convenient for isolating aldolase on a large scale from other mammalian and avian sources. Aldolase from both human liver and muscle was crystallized; each gave two crystalline forms, depending on the conditions of crystallization. X-ray studies on the muscle aldolase crystals suggest a close structural similarity between human and rabbit muscle aldolase. Aldolases from human muscle and liver have similar pH optima and pH stability but their stability to heat treatment differs. The effect of heat on the enzymes may therefore provide an easy means of distinguishing them. The kinetic constants Km and kcat. for these aldolases are similar to other mammalian aldolases. Amino acid analyses and tryptic peptide `mapping' show that the primary structures of the two aldolases differ greatly.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J., Gibbons I., Perham R. N. A comparative study of the structure of muscle fructose 1,6-diphosphate aldolases. Eur J Biochem. 1969 Dec;11(3):503–509. doi: 10.1111/j.1432-1033.1969.tb00802.x. [DOI] [PubMed] [Google Scholar]
- Anstall H. B., Lapp C., Trujillo J. M. Isozymes of aldolase. Science. 1966 Nov 4;154(3749):657–658. doi: 10.1126/science.154.3749.657. [DOI] [PubMed] [Google Scholar]
- Arnold H., Pette D. Binding of glycolytic enzymes to structure proteins of the muscle. Eur J Biochem. 1968 Nov;6(2):163–171. doi: 10.1111/j.1432-1033.1968.tb00434.x. [DOI] [PubMed] [Google Scholar]
- DRECHSLER E. R., BOYER P. D., KOWALSKY A. G. The catalytic activity of carboxypeptidase-degraded aldolase. J Biol Chem. 1959 Oct;234:2627–2634. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Eagles P. A., Johnson L. N., Joynson M. A., McMurray C. H., Gutfreund H. Subunit structure of aldolase: chemical and crystallographic evidence. J Mol Biol. 1969 Nov 14;45(3):533–544. doi: 10.1016/0022-2836(69)90310-6. [DOI] [PubMed] [Google Scholar]
- Forcina B. G., Perham R. N. Amino acid sequence homology between muscle and liver aldolases. FEBS Lett. 1971 Oct 15;18(1):59–63. doi: 10.1016/0014-5793(71)80406-4. [DOI] [PubMed] [Google Scholar]
- Gibbons I., Anderson P. J., Perham R. N. Amino acid sequence homology in the active site of rabbit and sturgeon muscle aldolases. FEBS Lett. 1970 Sep 18;10(1):49–53. doi: 10.1016/0014-5793(70)80413-6. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracy R. W., Lacko A. G., Brox L. W., Adelman R. C., Horecker B. L. Structural relations in aldolases purified from rat liver and muscle and Novikoff hepatoma. Arch Biochem Biophys. 1970 Feb;136(2):480–490. doi: 10.1016/0003-9861(70)90219-5. [DOI] [PubMed] [Google Scholar]
- Gracy R. W., Lacko A. G., Horecker B. L. Subunit structure and chemical properties of rabbit liver aldolase. J Biol Chem. 1969 Jul 25;244(14):3913–3919. [PubMed] [Google Scholar]
- Gürtler B., Bally C., Leuthardt F. Reindarstellung und Eigenschaften der menschlichen Leberaldolase. 19. Uber Aldolasen. Hoppe Seylers Z Physiol Chem. 1971 Oct;352(10):1455–1462. [PubMed] [Google Scholar]
- Gürtler B., Leuthardt F. Darstellung reiner Aldolase aus menschlicher Leber. 17. Uber Aldolasen. Hoppe Seylers Z Physiol Chem. 1969 Jul;350(7):915–916. [PubMed] [Google Scholar]
- Ikehara Y., Endo H., Okada Y. The identity of the aldolases isolated from rat muscle and primary hepatoma. Arch Biochem Biophys. 1970 Feb;136(2):491–497. doi: 10.1016/0003-9861(70)90220-1. [DOI] [PubMed] [Google Scholar]
- KATZ R., DUCCI H. Serum aldolase in hepatobiliary disease. Am J Dig Dis. 1958 Jul;3(7):517–521. doi: 10.1007/BF02231251. [DOI] [PubMed] [Google Scholar]
- Kawahara K., Tanford C. The number of polypeptide chains in rabbit muscle aldolase. Biochemistry. 1966 May;5(5):1578–1584. doi: 10.1021/bi00869a018. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacko A. G., Brox L. W., Gracy R. W., Horecker B. L. The carboxyl-terminal structure of rabbit liver aldolase (aldolase B). J Biol Chem. 1970 Apr 25;245(8):2140–2141. [PubMed] [Google Scholar]
- Lai C. Y., Chen C. Studies on the structure of rabbit muscle aldolase. II. Primary structure of peptide CnV and chemical evidence for the four-subunit structure of aldolase. Arch Biochem Biophys. 1968 Oct;128(1):212–218. doi: 10.1016/0003-9861(68)90024-6. [DOI] [PubMed] [Google Scholar]
- Lebherz H. G., Rutter W. J. Distribution of fructose diphosphate aldolase variants in biological systems. Biochemistry. 1969 Jan;8(1):109–121. doi: 10.1021/bi00829a016. [DOI] [PubMed] [Google Scholar]
- Marquardt R. R. Multiple molecular forms of avian aldolases. I. Crystallization and physical properties of chicken (Gallus domesticus) breast muscle aldolase. Can J Biochem. 1969 May;47(5):517–526. doi: 10.1139/o69-081. [DOI] [PubMed] [Google Scholar]
- Matsushima T., Kawabe S., Sugimura T. Crystallization of rat liver aldolase. J Biochem. 1968 Apr;63(4):555–557. doi: 10.1093/oxfordjournals.jbchem.a128811. [DOI] [PubMed] [Google Scholar]
- NORBERG B. Serum enzyme studies in some icteric conditions. Clin Chim Acta. 1961 Mar;6:264–271. doi: 10.1016/0009-8981(61)90093-6. [DOI] [PubMed] [Google Scholar]
- Nordmann Y., Schapira F. Muscle type isoenzymes of liver aldolase in hepatomas. Eur J Cancer. 1967 Nov;3(4):247–250. doi: 10.1016/0014-2964(67)90004-7. [DOI] [PubMed] [Google Scholar]
- PEANASKY R. J., LARDY H. A. Bovine liver aldolase. I. Isolation, crystallization, and some general properties. J Biol Chem. 1958 Aug;233(2):365–370. [PubMed] [Google Scholar]
- Penhoet E., Kochman M., Valentine R., Rutter W. J. The subunit structure of mammalian fructose diphosphate aldolase. Biochemistry. 1967 Sep;6(9):2940–2949. doi: 10.1021/bi00861a039. [DOI] [PubMed] [Google Scholar]
- Penhoet E., Rajkumar T., Rutter W. J. Multiple forms of fructose diphosphate aldolase in mammalian tissues. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1275–1282. doi: 10.1073/pnas.56.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman S. J., Coulson A. F., Farley I. R., Riddleston B., Knowles J. R. Specificity and kinetics of triose phosphate isomerase from chicken muscle. Biochem J. 1972 Sep;129(2):301–310. doi: 10.1042/bj1290301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWELL N. R., SMITH A. J. Multiple serial enzyme studies in acute myocardial infarction. Br Med J. 1959 Sep 19;2(5150):459–463. doi: 10.1136/bmj.2.5150.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUTTER W. J., BLOSTEIN R. E., WOODFIN B. M., WEBER C. S. ENZYME VARIANTS AND METABOLIC DIVERSIFICATION. Adv Enzyme Regul. 1963;1:39–56. doi: 10.1016/0065-2571(63)90005-0. [DOI] [PubMed] [Google Scholar]
- SCHAPIRA F., DREYFUS J. C., SCHAPIRA G. ANOMALY OF ALDOLASE IN PRIMARY LIVER CANCER. Nature. 1963 Dec 7;200:995–997. doi: 10.1038/200995a0. [DOI] [PubMed] [Google Scholar]
- SCHAPIRA F. [Normal aldolase activity of cerebrospinal fluid]. Clin Chim Acta. 1962 Jul;7:566–570. doi: 10.1016/0009-8981(62)90101-8. [DOI] [PubMed] [Google Scholar]
- SIBLEY J. A., FLEISHER G. A. The clinical significance of serum aldolase. Proc Staff Meet Mayo Clin. 1954 Nov 10;29(23):591–603. [PubMed] [Google Scholar]
- Sia C. L., Horecker B. L. The molecular weight of rabbit muscle aldolase and the properties of the subunits in acid solution. Arch Biochem Biophys. 1968 Jan;123(1):186–194. doi: 10.1016/0003-9861(68)90118-5. [DOI] [PubMed] [Google Scholar]
- Steinman H. M., Richards F. M. Participation of cysteinyl residues in the structure and function of muscle aldolase. Characterization of mixed disulfide derivatives. Biochemistry. 1970 Oct 27;9(22):4360–4372. doi: 10.1021/bi00824a017. [DOI] [PubMed] [Google Scholar]
- Szajáni B., Sajgó M., Biszku E., Friedrich P., Szabolcsi G. Identification of a cysteinyl residue involved in the activity of rabbit muscle aldolase. Eur J Biochem. 1970 Jul;15(1):171–178. doi: 10.1111/j.1432-1033.1970.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Ting S. M., Sia C. L., Lai C. Y., Horecker B. L. Frog muscle aldolase: purification of the enzyme and structure of the active site. Arch Biochem Biophys. 1971 Jun;144(2):485–490. doi: 10.1016/0003-9861(71)90352-3. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]