Abstract
1. The general applicability of affinity chromatography to the purification of nicotinamide nucleotide-dependent dehydrogenases on immobilized cofactors is illustrated with several examples taken from crude systems. 2. Methods for overcoming the inevitable loss of selectivity experienced with these polymers are suggested. Effective use of the appropriate nucleotide, the second substrate and other interacting ligands can be made to selectively alter the chromatographic behaviour of the desired enzyme.
Full text
PDF
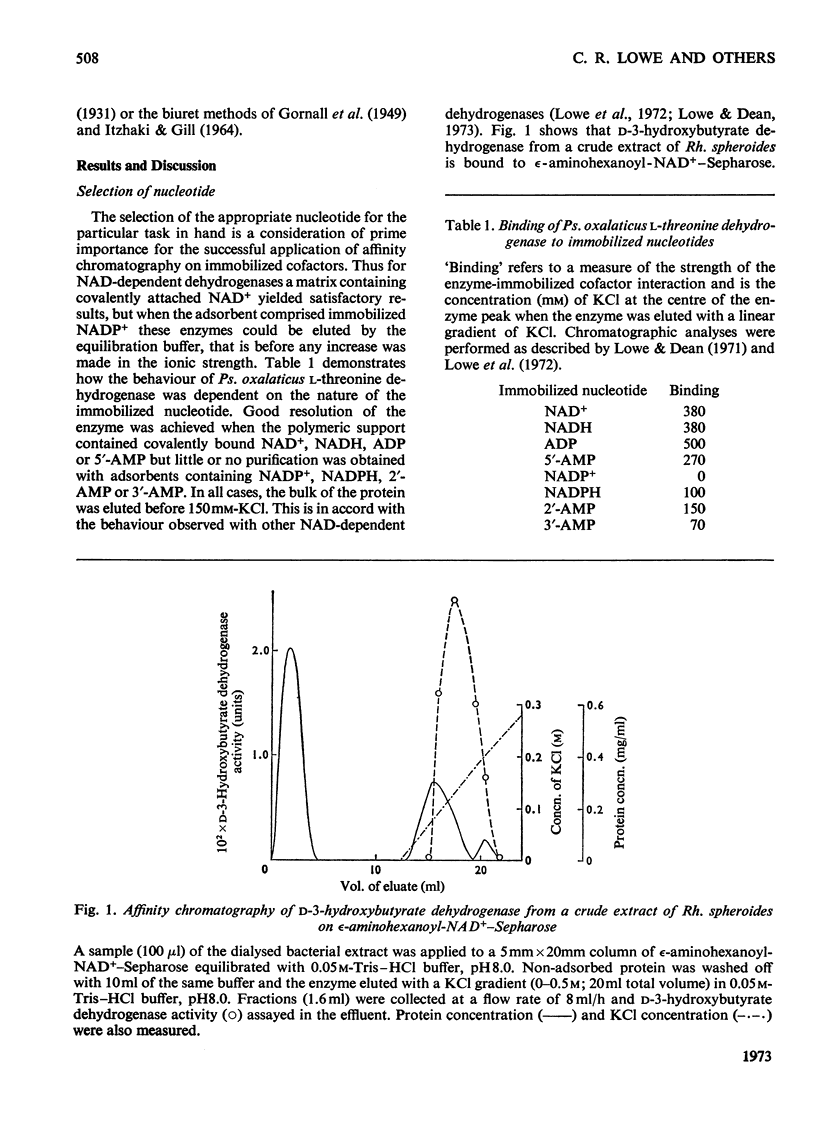


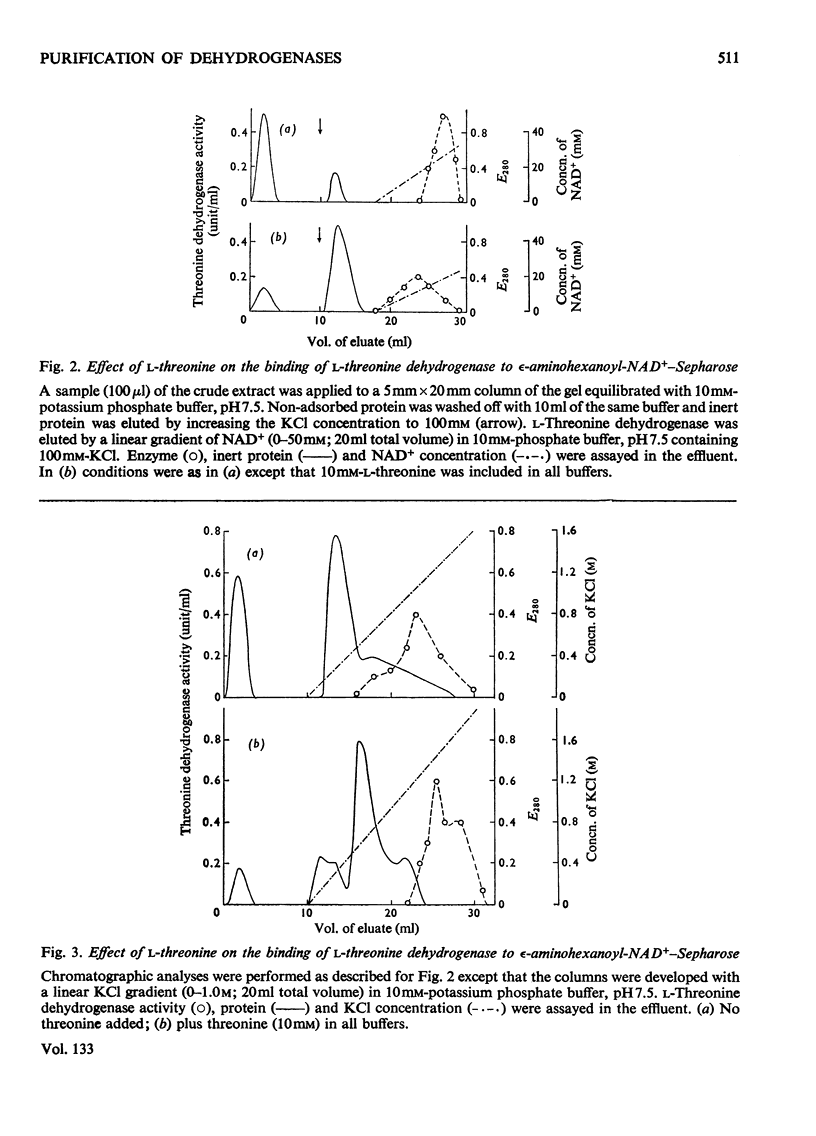
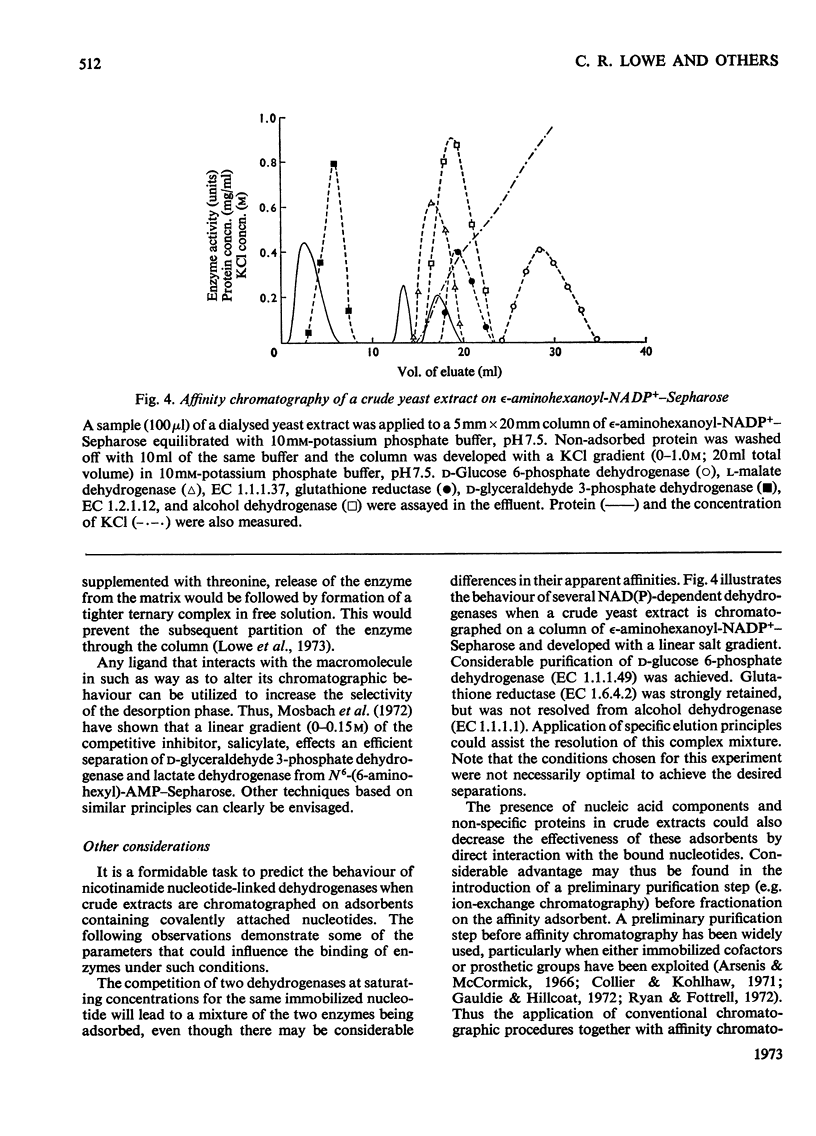

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arsenis C., McCormick D. B. Purification of flavin mononucleotide-dependent enzymes by column chromatography on flavin phosphate cellulose compounds. J Biol Chem. 1966 Jan 25;241(2):330–334. [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bergmeyer H. U., Gawehn K., Klotzsch H., Krebs H. A., Williamson D. H. Purification and properties of crystalline 3-hydroxybutyrate dehydrogenase from Rhodopseudomonas spheroides. Biochem J. 1967 Feb;102(2):423–431. doi: 10.1042/bj1020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore M. A., Turner J. M. Threonine metabolism via two-carbon compounds by Pseudomonas oxalaticus. J Gen Microbiol. 1971 Aug;67(2):243–246. doi: 10.1099/00221287-67-2-243. [DOI] [PubMed] [Google Scholar]
- Collier R., Kohlhaw G. Affinity chromatography of transaminases. Anal Biochem. 1971 Jul;42(1):48–53. doi: 10.1016/0003-2697(71)90008-x. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography. Annu Rev Biochem. 1971;40:259–278. doi: 10.1146/annurev.bi.40.070171.001355. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterby J. S., Rosemeyer M. A. Purification and subunit interactions of yeast hexokinase. Eur J Biochem. 1972 Jul 13;28(2):241–252. doi: 10.1111/j.1432-1033.1972.tb01907.x. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Hillcoat B. L. Purification of tetrahydrofolate dehydrogenase by affinity chromatography. Biochim Biophys Acta. 1972 Apr 7;268(1):35–40. doi: 10.1016/0005-2744(72)90194-5. [DOI] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Kaufman B. T., Pierce J. V. Purification of dihydrofolic reductase from chicken liver by affinity chromatography. Biochem Biophys Res Commun. 1971 Aug 6;44(3):608–613. doi: 10.1016/s0006-291x(71)80126-2. [DOI] [PubMed] [Google Scholar]
- Larsson P. O., Mosbach K. Preparation of a NAD(H)-polymer matrix showing coenzyme function of the bound pyridine nucleotide. Biotechnol Bioeng. 1971 May;13(3):393–398. doi: 10.1002/bit.260130306. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Dean P. D. Affinity chromatography of lactate dehydrogenase on immobilized nucleotides. Biochem J. 1973 Jul;133(3):515–520. doi: 10.1042/bj1330515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C. R., Dean P. D.G. Affinity chromatography of enzymes on insolubilized cofactors. FEBS Lett. 1971 May 20;14(5):313–316. doi: 10.1016/0014-5793(71)80288-0. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Harvey M. J., Craven D. B., Dean P. D. Some parameters relevant to affinity chromatography on immobilized nucleotides. Biochem J. 1973 Jul;133(3):499–506. doi: 10.1042/bj1330499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C. R., Mosbach K., Dean P. D. Some applications of insolubilised cofactors to the purification of pyridine nucleotide-dependent dehydrogenases. Biochem Biophys Res Commun. 1972 Aug 21;48(4):1004–1010. doi: 10.1016/0006-291x(72)90708-5. [DOI] [PubMed] [Google Scholar]
- Mosbach K., Guilford H., Ohlsson R., Scott M. General ligands in affinity chromatography. Cofactor-substrate elution of enzymes bound to the immobilized nucleotides adenosine 5'-monophosphate and nicotinamide-adenine dinucleotide. Biochem J. 1972 May;127(4):625–631. doi: 10.1042/bj1270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carra P., Barry S. Affinity chromatography of lactate dehydrogenase Model studies demonstrating the potential of the technique in the mechanistic investigation as well as in the purification of multi-substrate enzymes. FEBS Lett. 1972 Apr 1;21(3):281–285. doi: 10.1016/0014-5793(72)80183-2. [DOI] [PubMed] [Google Scholar]
- Ryan E., Fottrell P. F. Interactions between an unusual aspartate aminotransferase from Rhizobium japonicum and pyridoxal-5'-phosphate studied by affinity chromatography. FEBS Lett. 1972 Jun 1;23(1):73–76. doi: 10.1016/0014-5793(72)80288-6. [DOI] [PubMed] [Google Scholar]


