Abstract
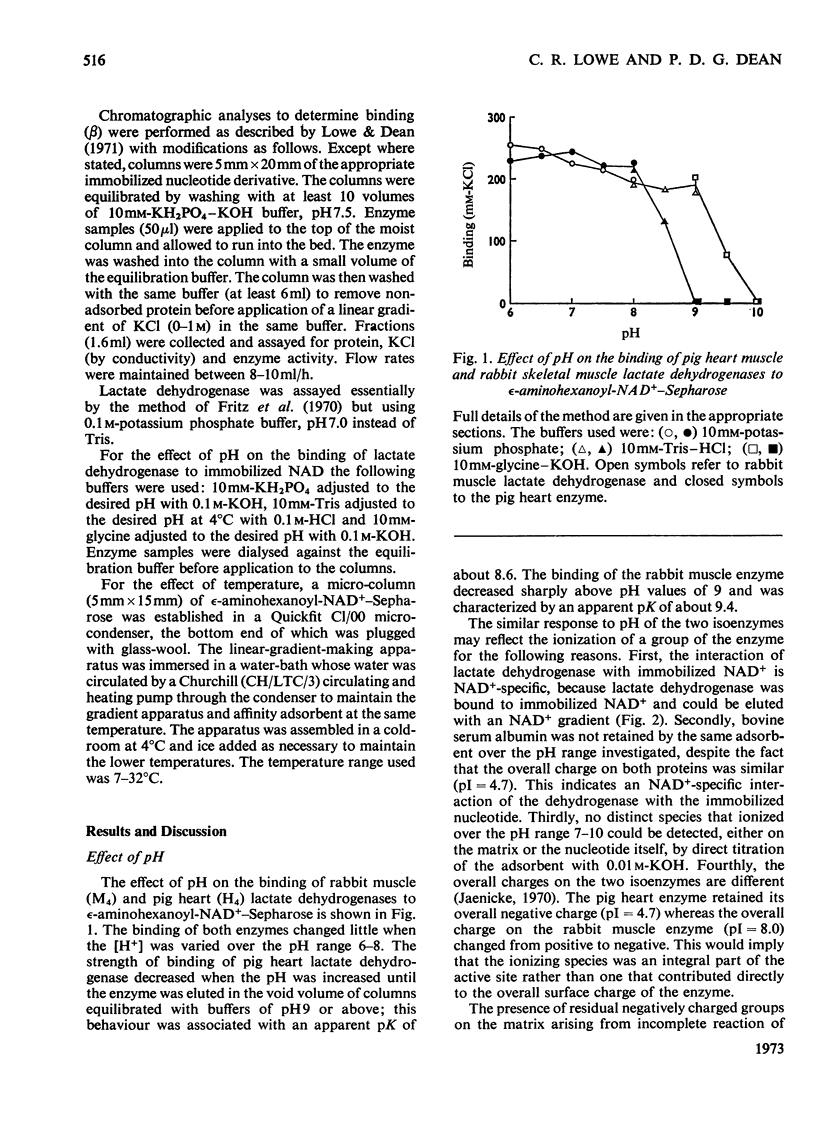
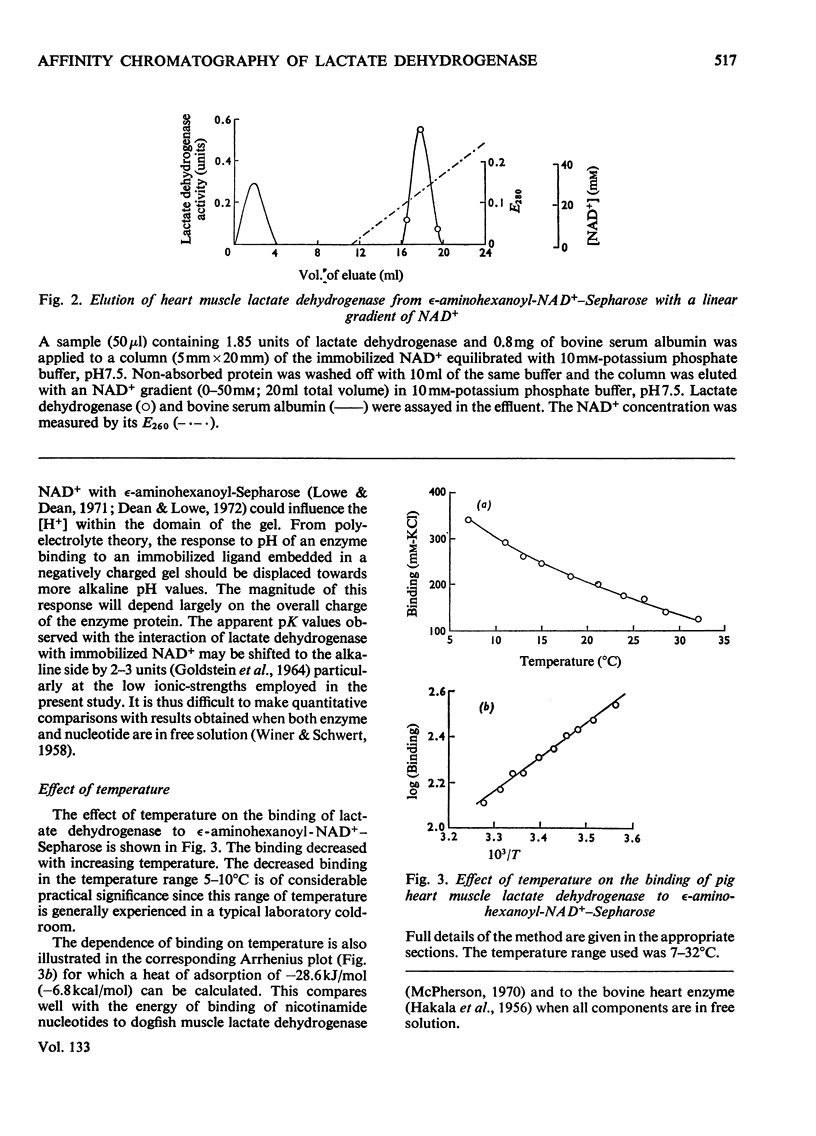
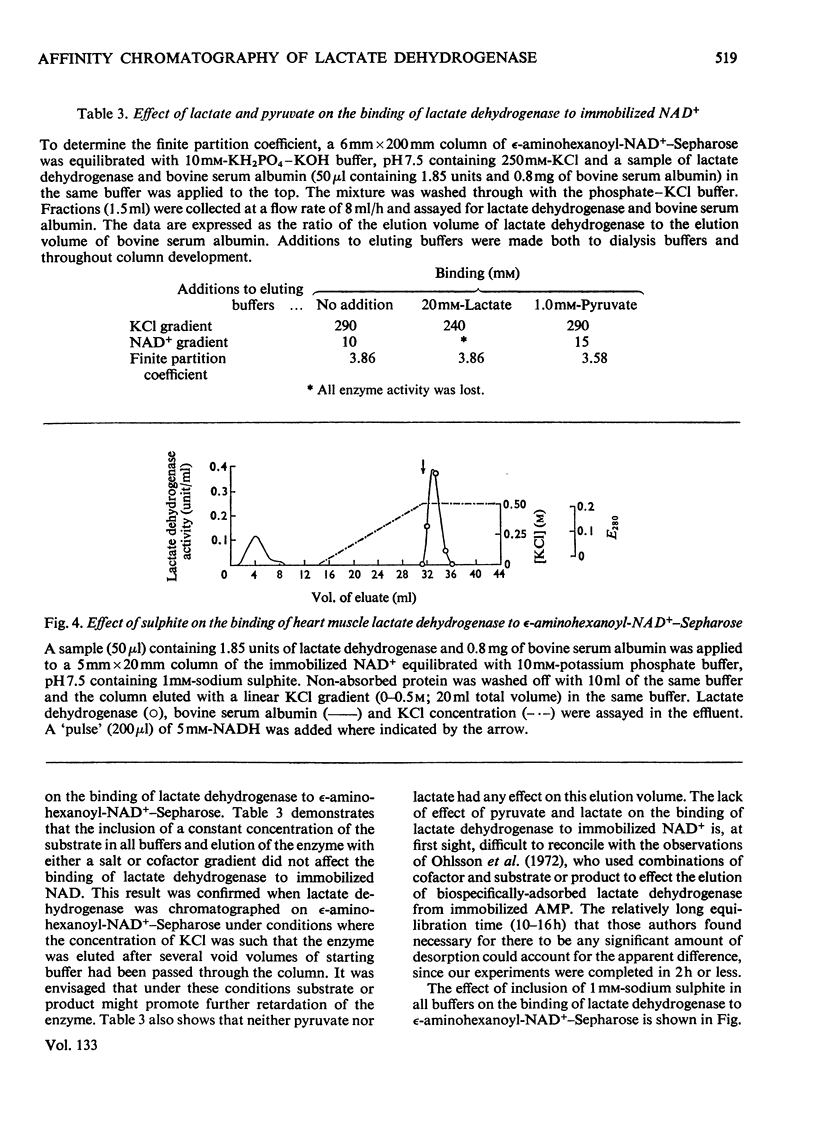
The interaction of two isoenzymes of lactate dehydrogenase from pig heart muscle (H4) and rabbit skeletal muscle (M4), with immobilized nucleotides was examined: the effects of pH and temperature on the binding of lactate dehydrogenase were studied with immobilized NAD+ matrices. The influence of substrate, product and sulphite on the binding of heart muscle lactate dehydrogenase to immobilized NAD+ was investigated. The interaction of both lactate dehydrogenase isoenzymes with immobilized pyridine and adenine nucleotides and their derivatives were measured. The effects of these parameters on the interaction of lactate dehydrogenase with immobilized nucleotides were correlated with the known kinetic and molecular properties of the enzymes in free solution.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akanuma H., Kasuga A., Akanuma T., Yamasaki M. The effective use of affinity chromatogrphy for the study of complex formation of bovine carboxypeptidase B with basic and aromatic amino acid analogues. Biochem Biophys Res Commun. 1971 Oct 1;45(1):27–33. doi: 10.1016/0006-291x(71)90045-3. [DOI] [PubMed] [Google Scholar]
- Anderson R. G., Hussey H., Baddiley J. The mechanism of wall synthesis in bacteria. The organization of enzymes and isoprenoid phosphates in the membrane. Biochem J. 1972 Mar;127(1):11–25. doi: 10.1042/bj1270011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenis C., McCormick D. B. Purification of flavin mononucleotide-dependent enzymes by column chromatography on flavin phosphate cellulose compounds. J Biol Chem. 1966 Jan 25;241(2):330–334. [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Collier R., Kohlhaw G. Affinity chromatography of transaminases. Anal Biochem. 1971 Jul;42(1):48–53. doi: 10.1016/0003-2697(71)90008-x. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography. Annu Rev Biochem. 1971;40:259–278. doi: 10.1146/annurev.bi.40.070171.001355. [DOI] [PubMed] [Google Scholar]
- Edmondson D., Massey V., Palmer G., Beacham L. M., 3rd, Elion G. B. The resolution of active and inactive xanthine oxidase by affinity chromatography. J Biol Chem. 1972 Mar 10;247(5):1597–1604. [PubMed] [Google Scholar]
- Fritz P. J., Morrison W. J., White E. L., Vesell E. S. Comparative study of methods for quantitation of lactate dehydrogenase isozymes. Anal Biochem. 1970 Aug;36(2):443–453. doi: 10.1016/0003-2697(70)90381-7. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN L., LEVIN Y., KATCHALSKI E. A WATER-INSOLUBLE POLYANIONIC DERIVATIVE OF TRYPSIN. II. EFFECT OF THE POLYELECTROLYTE CARRIER ON THE KINETIC BEHAVIOR OF THE BOUND TRYPSIN. Biochemistry. 1964 Dec;3:1913–1919. doi: 10.1021/bi00900a022. [DOI] [PubMed] [Google Scholar]
- Gawronski T. H., Wold F. A study of peptide-peptide interactions on an insoluble matrix. Biochemistry. 1972 Feb 1;11(3):442–448. doi: 10.1021/bi00753a023. [DOI] [PubMed] [Google Scholar]
- HAKALA M. T., GLAID A. J., SCHWERT G. W. Lactic dehydrogenase. II. Variation of kinetic and equilibrium constants with temperature. J Biol Chem. 1956 Jul;221(1):191–209. [PubMed] [Google Scholar]
- Holbrook J. J. The importance of SH-groups for enzymic activity. V. The coenzyme-binding capacity of pig heart lactate dehydrogenase, isozyme I, after inhibition by various maleinimides. Biochem Z. 1966 Mar 28;344(2):141–152. [PubMed] [Google Scholar]
- Larsson P. O., Mosbach K. Preparation of a NAD(H)-polymer matrix showing coenzyme function of the bound pyridine nucleotide. Biotechnol Bioeng. 1971 May;13(3):393–398. doi: 10.1002/bit.260130306. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Dean P. D.G. Affinity chromatography of enzymes on insolubilized cofactors. FEBS Lett. 1971 May 20;14(5):313–316. doi: 10.1016/0014-5793(71)80288-0. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Harvey M. J., Craven D. B., Dean P. D. Some parameters relevant to affinity chromatography on immobilized nucleotides. Biochem J. 1973 Jul;133(3):499–506. doi: 10.1042/bj1330499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C. R., Mosbach K., Dean P. D. Some applications of insolubilised cofactors to the purification of pyridine nucleotide-dependent dehydrogenases. Biochem Biophys Res Commun. 1972 Aug 21;48(4):1004–1010. doi: 10.1016/0006-291x(72)90708-5. [DOI] [PubMed] [Google Scholar]
- McPherson A., Jr Interaction of lactate dehydrogenase with its coenzyme, nicotinamide-adenine dinucleotide. J Mol Biol. 1970 Jul 14;51(1):39–46. doi: 10.1016/0022-2836(70)90268-8. [DOI] [PubMed] [Google Scholar]
- Mosbach K., Guilford H., Ohlsson R., Scott M. General ligands in affinity chromatography. Cofactor-substrate elution of enzymes bound to the immobilized nucleotides adenosine 5'-monophosphate and nicotinamide-adenine dinucleotide. Biochem J. 1972 May;127(4):625–631. doi: 10.1042/bj1270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson R., Brodelius P., Mosbach K. Affinity chromatography of enzymes on an AMP-analogue: Specific elution of dehydrogenases from a general ligand. FEBS Lett. 1972 Sep 15;25(2):234–238. doi: 10.1016/0014-5793(72)80492-7. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Brostrom C. O., Corbin J. D., King C. A., Krebs E. G. Separation of regulatory and catalytic subunits of the cyclic 3',5'-adenosine monophosphate-dependent protein kinase(s) of rabbit skeletal muscle. Biochem Biophys Res Commun. 1971 Jan 22;42(2):187–194. doi: 10.1016/0006-291x(71)90086-6. [DOI] [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W. Lactic dehydrogenase. IV. The influence of pH on the kinetics of the reaction. J Biol Chem. 1958 Apr;231(2):1065–1083. [PubMed] [Google Scholar]


