Abstract
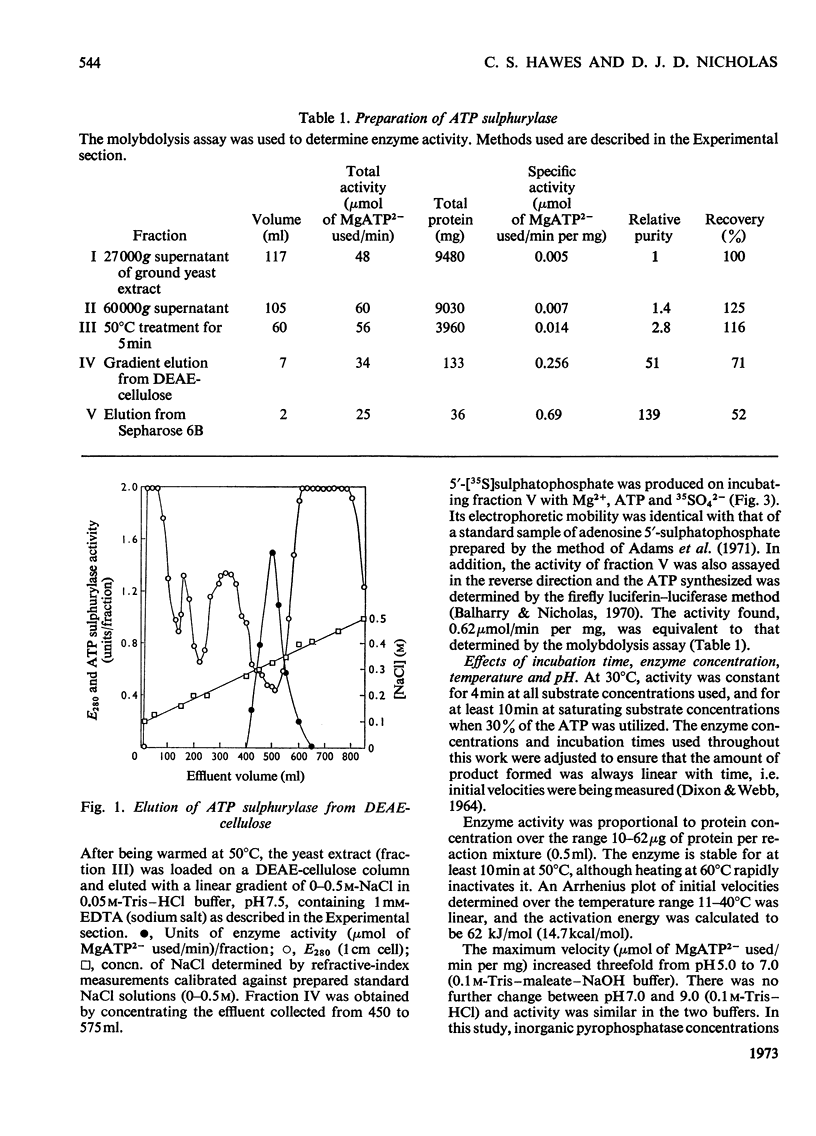
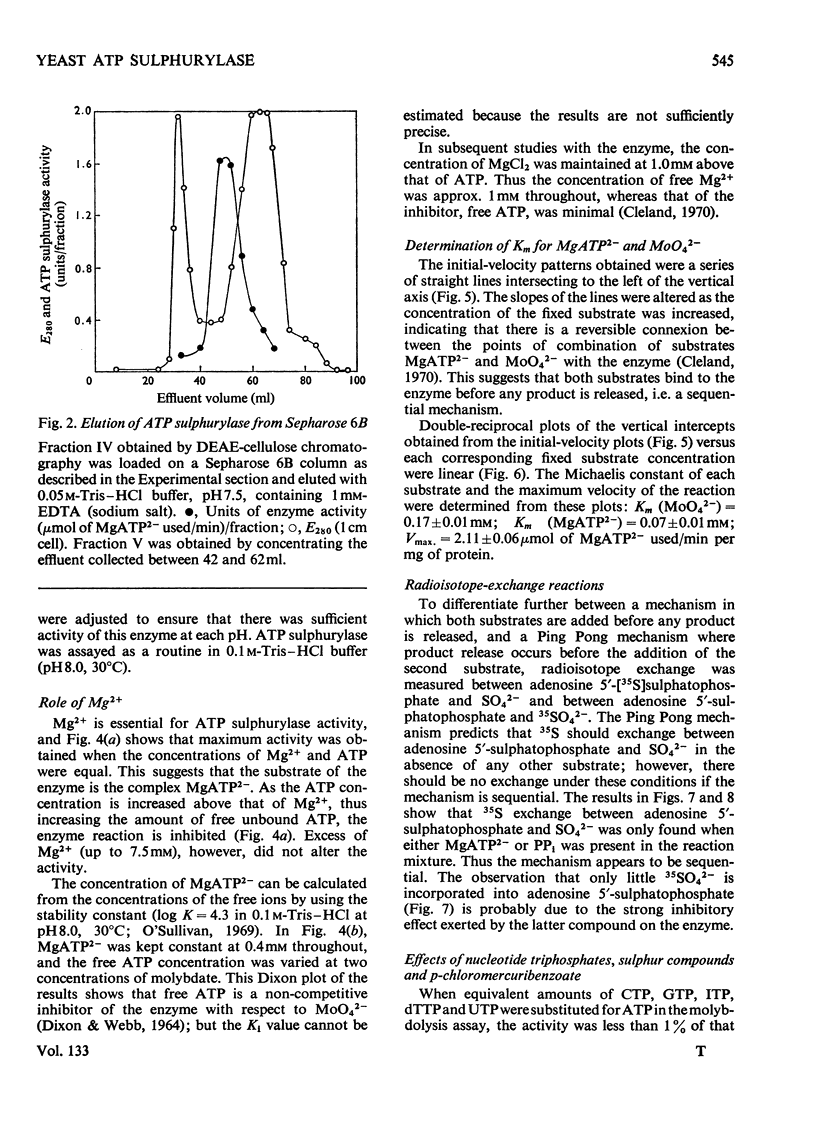
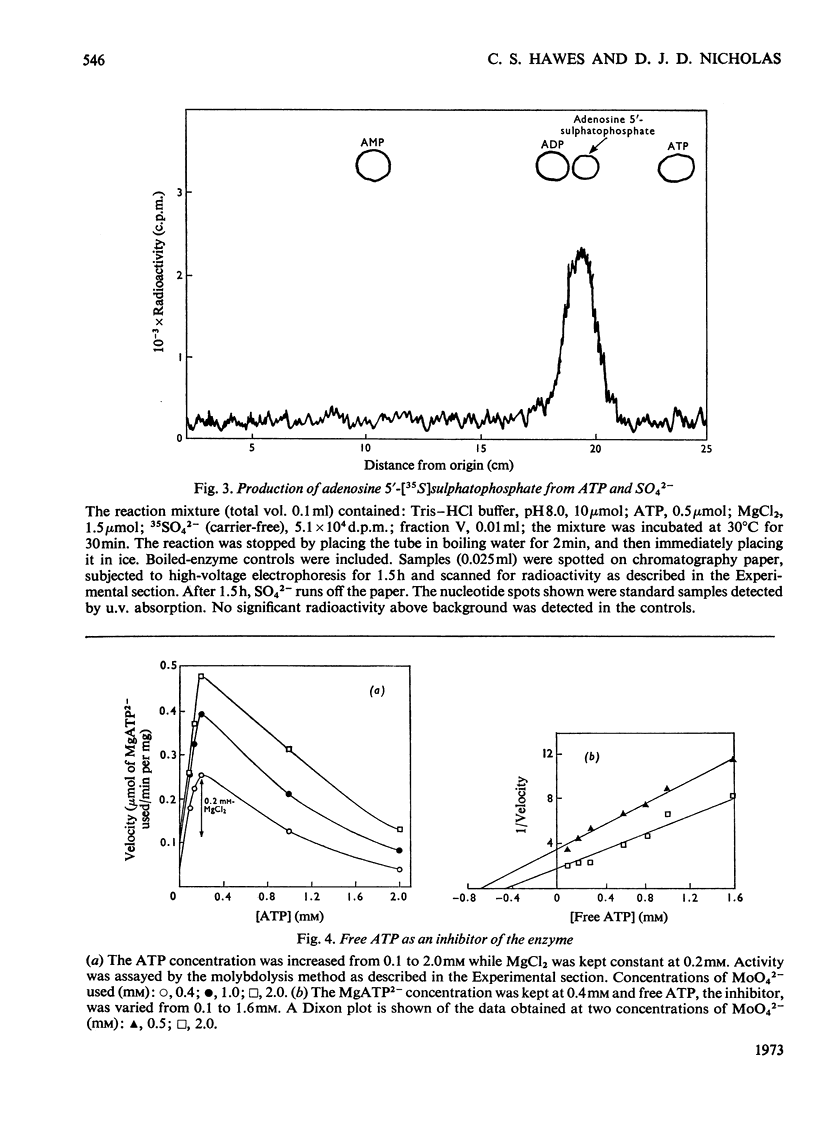
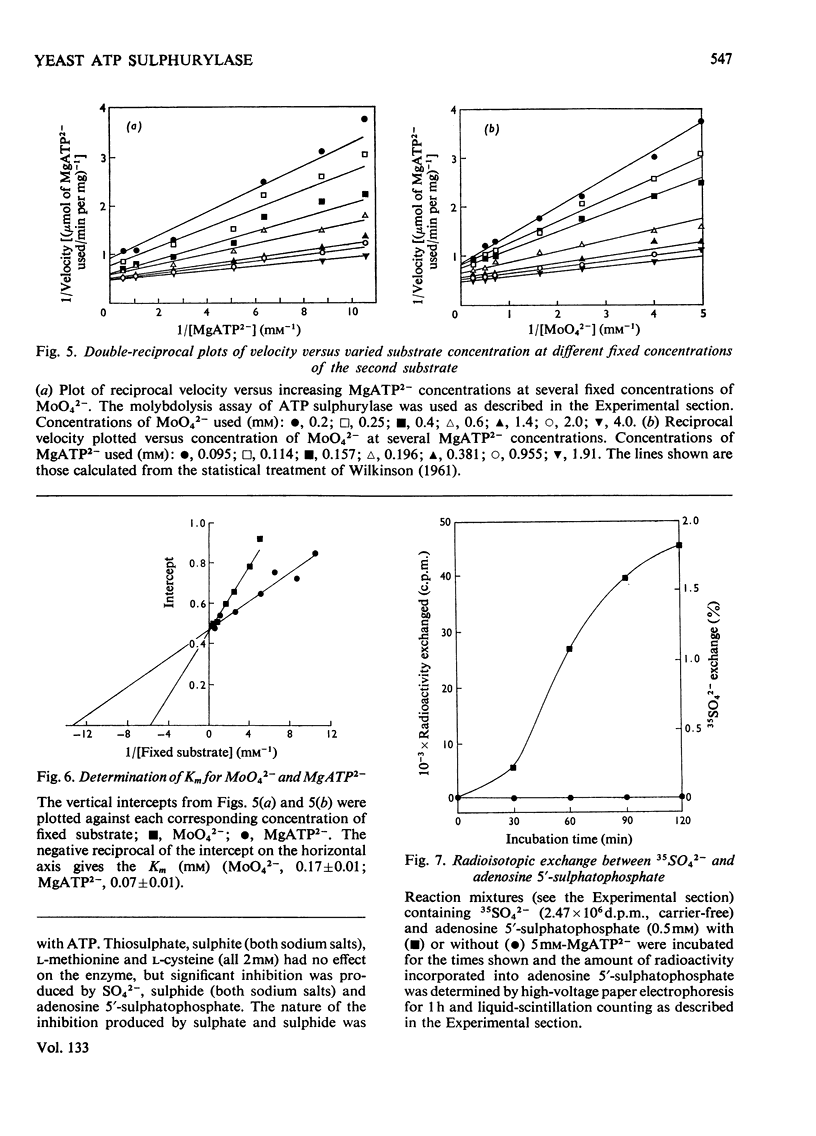
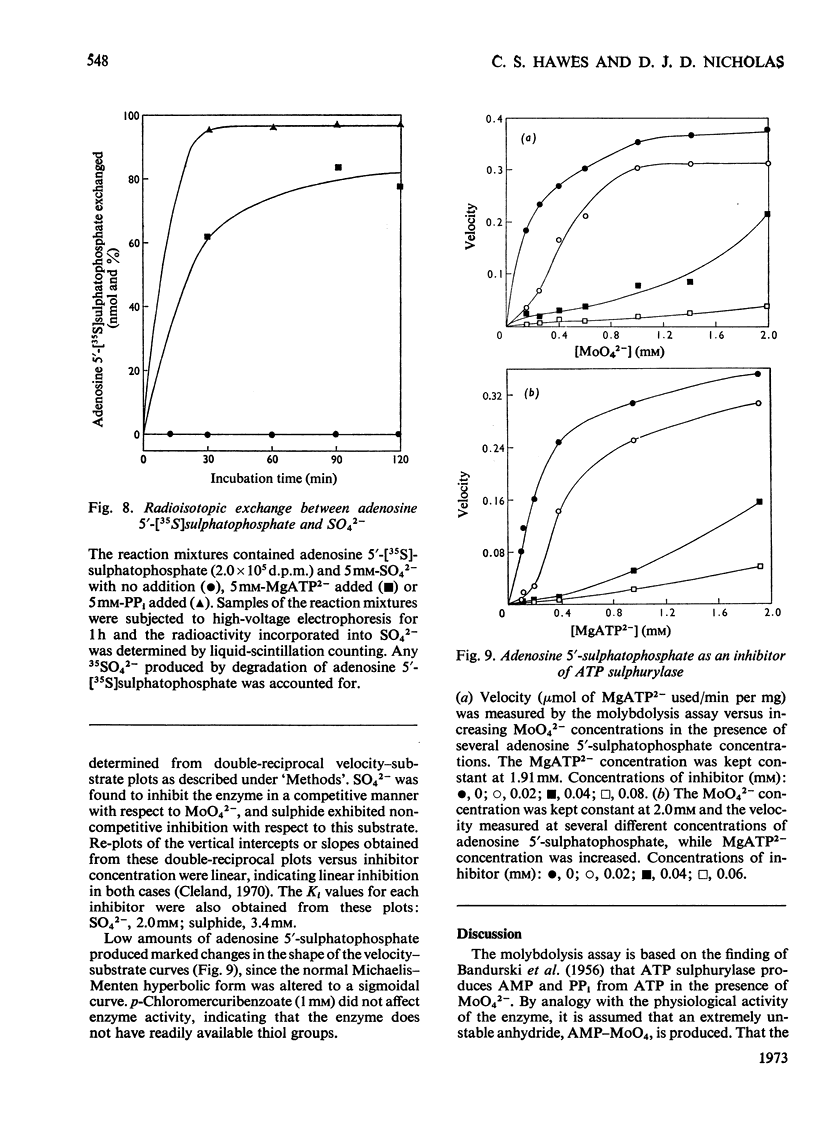
1. ATP sulphurylase from Saccharomyces cerevisiae was purified 140-fold by using heat treatment, DEAE-cellulose chromatography and Sepharose 6B gel filtration. 2. The enzyme was stable at −15°C, optimum reaction velocity was between pH7.0 and 9.0, and the activation energy was 62kJ/mol (14.7kcal/mol). 3. The substrate was shown to be the MgATP2− complex, free ATP being inhibitory. 4. Double-reciprocal plots from initial-velocity studies were intersecting and the Km of each substrate was determined at infinite concentration of the other (Km MgATP2−, 0.07mm; MoO42−, 0.17mm). 5. Radio-isotopic exchange between the substrate pairs, adenosine 5′-[35S]sulphatophosphate and SO42−, 35SO42− and adenosine 5′-sulphatophosphate, occurred only in the presence of either MgATP2− or PPi. This suggests, along with the initial-velocity data, a sequential reaction mechanism in which both substrates bind before any product is released. 6. The enzyme reaction was specific for ATP and was not inhibited by l-cysteine, l-methionine, SO32−, S2O32− (all 2mm) nor by p-chloromercuribenzoate (1mm). 7. Competitive inhibition of the enzyme with respect to MoO42− was produced by SO42− (Ki=2.0mm) and non-competitive inhibition by sulphide (Ki=3.4mm). 8. Adenosine 5′-sulphatophosphate inhibited strongly and concentrations as low as 0.02mm altered the normal hyperbolic velocity–substrate curves with both MgATP2− and MoO42− to sigmoidal forms.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. A., Johnson R. E. ATP Sulfurylase Activity in the Soybean [Glycine max (L.) Merr.]. Plant Physiol. 1968 Dec;43(12):2041–2044. doi: 10.1104/pp.43.12.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams C. A., Warnes G. M., Nicholas D. J. Preparation of labeled adenosine 5'-phosphosulfate using APS reductase from Thibacillus denitrificans. Anal Biochem. 1971 Jul;42(1):207–213. doi: 10.1016/0003-2697(71)90028-5. [DOI] [PubMed] [Google Scholar]
- BALIGA B. S., VARTAK H. G., JAGANNATHAN V. Purification & propertis of sulfurylase from Desulovibrio desulfuricans. J Sci Ind Res (C) 1961 Feb;20C:33–40. [PubMed] [Google Scholar]
- Balharry G. J., Nicholas D. J. ATP-sulphurylase in spinach leaves. Biochim Biophys Acta. 1970 Dec 16;220(3):513–524. doi: 10.1016/0005-2744(70)90282-2. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Levi A. S., Wolf G. Purification and properties of the enzyme ATP-sulfurylase and its relation to vitamin A. Biochim Biophys Acta. 1969 Apr 22;178(2):262–282. doi: 10.1016/0005-2744(69)90395-7. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Enzymatic synthesis of adenosine-5'-phosphosulfate. J Biol Chem. 1958 Sep;233(3):686–690. [PubMed] [Google Scholar]
- SEGAL H. L. Sulfate-dependent exchange of pyrophosphate with nucleotide phosphate. Biochim Biophys Acta. 1956 Jul;21(1):194–195. doi: 10.1016/0006-3002(56)90125-1. [DOI] [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Assay of adenosine 5-triphosphate sulfurylase by pyrophosphate exchange. Plant Physiol. 1971 Jan;47(1):114–118. doi: 10.1104/pp.47.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Purification, properties and substrate specificity of adenosine triphosphate sulphurylase from spinach leaf tissue. Biochem J. 1972 Mar;127(1):237–247. doi: 10.1042/bj1270237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Marx W. Enzyme-substrate complexes of ATP-sulfurylase from mouse mastocytoma. Biochim Biophys Acta. 1972 Jan 20;258(1):125–132. doi: 10.1016/0005-2744(72)90972-2. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Su L. Y., Marx W. Purification and properties of ATP-sulfurylase from Furth mouse mastocytoma. Biochim Biophys Acta. 1972 Jan 20;258(1):113–124. doi: 10.1016/0005-2744(72)90971-0. [DOI] [PubMed] [Google Scholar]
- Tate M. E. Separation of myoinositol pentaphosphates by moving paper electrophoresis (MPE). Anal Biochem. 1968 Apr;23(1):141–149. doi: 10.1016/0003-2697(68)90019-5. [DOI] [PubMed] [Google Scholar]
- Tweedie J. W., Segel I. H. ATP-sulfurylase from Penicillium chrysogenum. I. Purification and characterization. Prep Biochem. 1971;1(2):91–117. doi: 10.1080/00327487108081932. [DOI] [PubMed] [Google Scholar]
- Tweedie J. W., Segel I. H. Adenosine triphosphate sulfurylase from Penicillium chrysogenum. II. Physical, kinetic, and regulatory properties. J Biol Chem. 1971 Apr 25;246(8):2438–2446. [PubMed] [Google Scholar]
- WHELDRAKE J. F., PASTERNAK C. A. THE CONTROL OF SULPHATE ACTIVATION IN BACTERIA. Biochem J. 1965 Jul;96:276–280. doi: 10.1042/bj0960276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]


